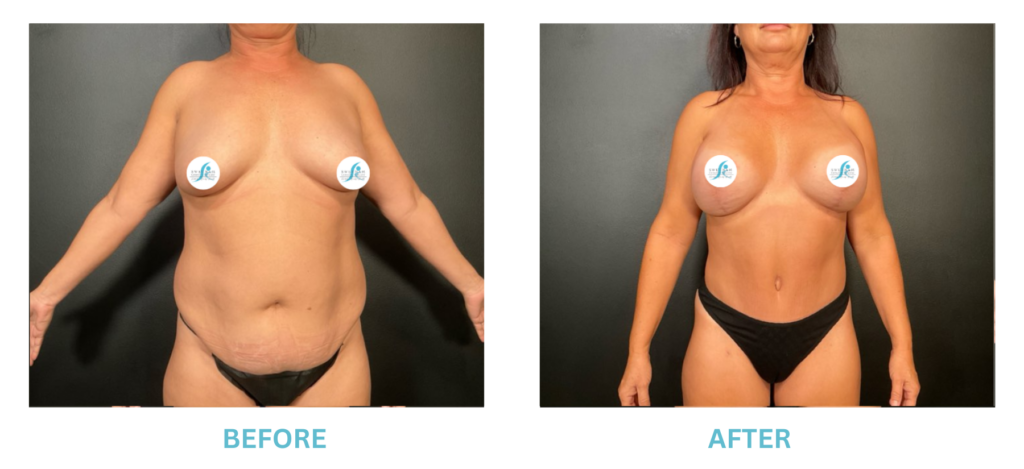
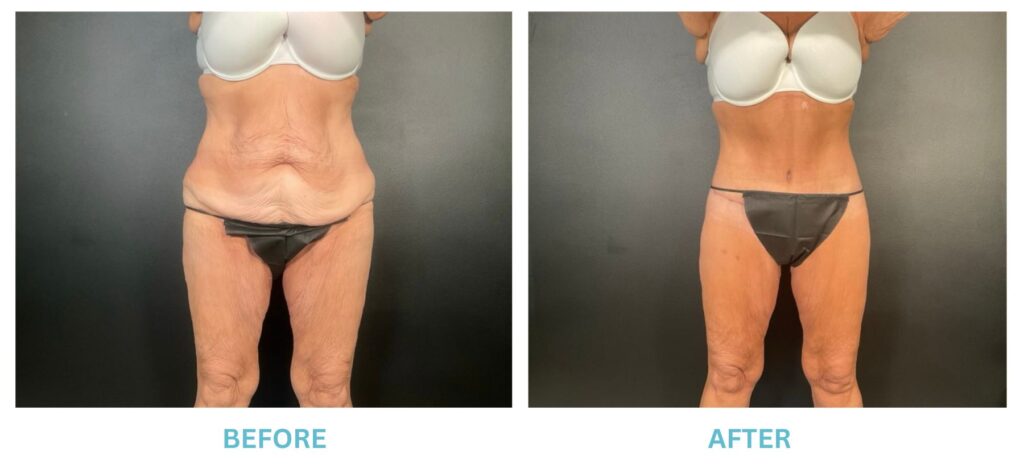
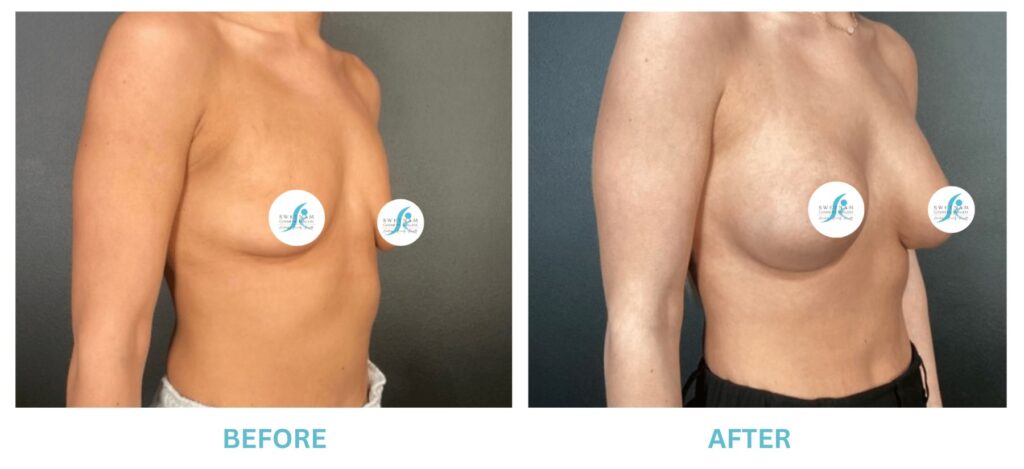
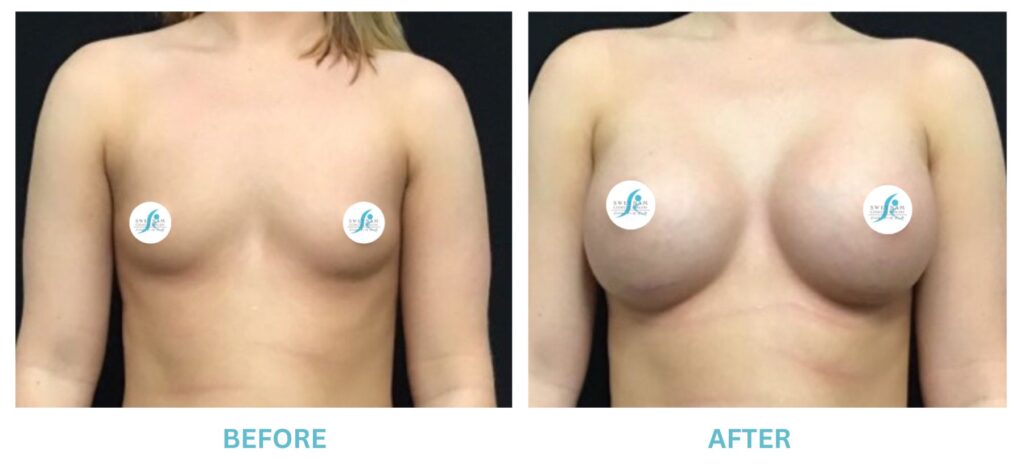
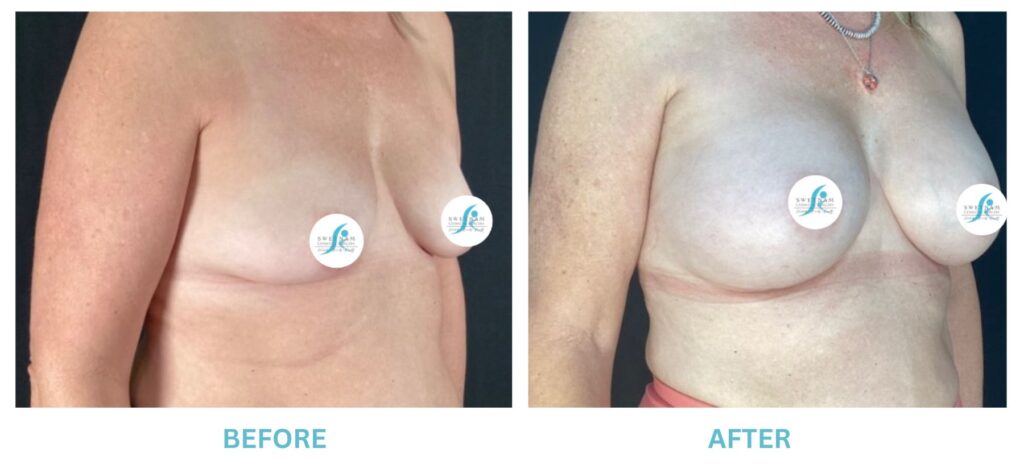
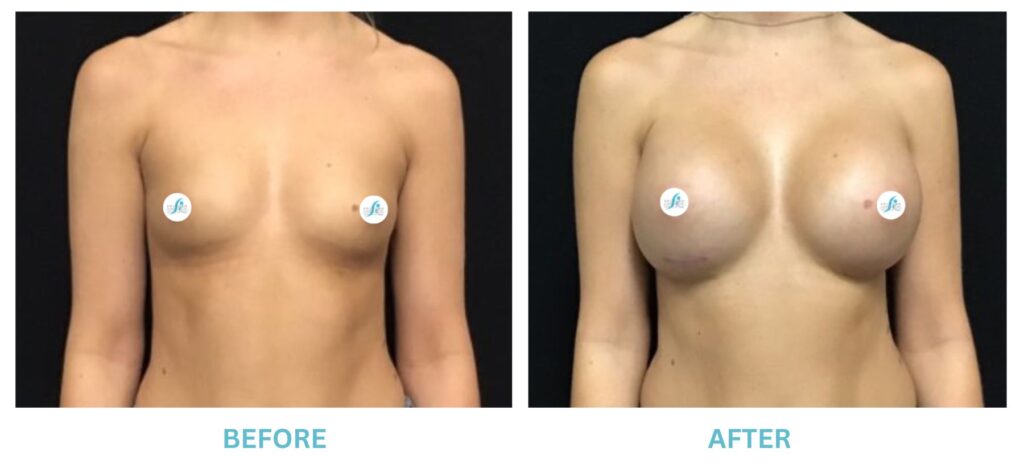
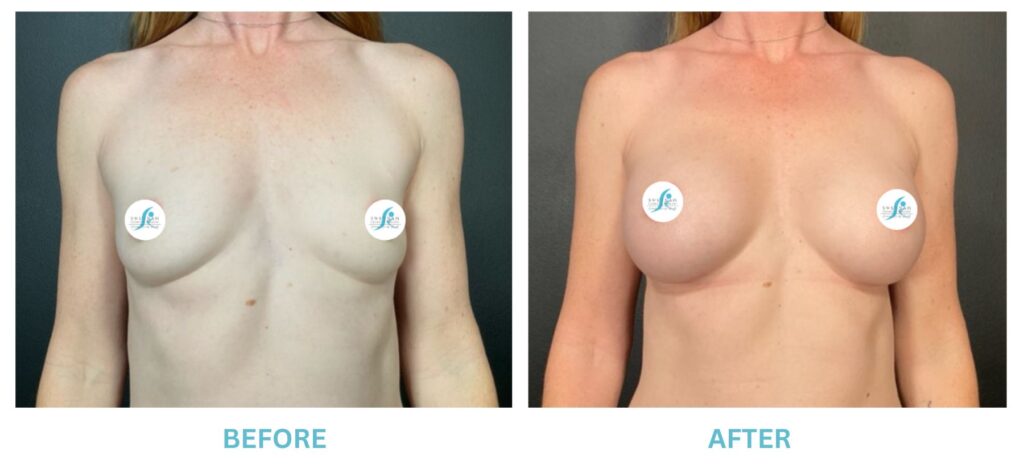
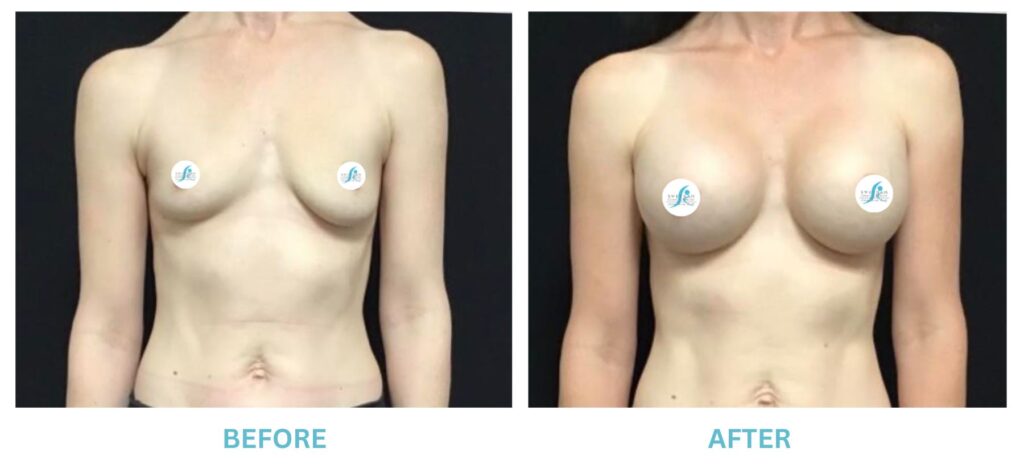
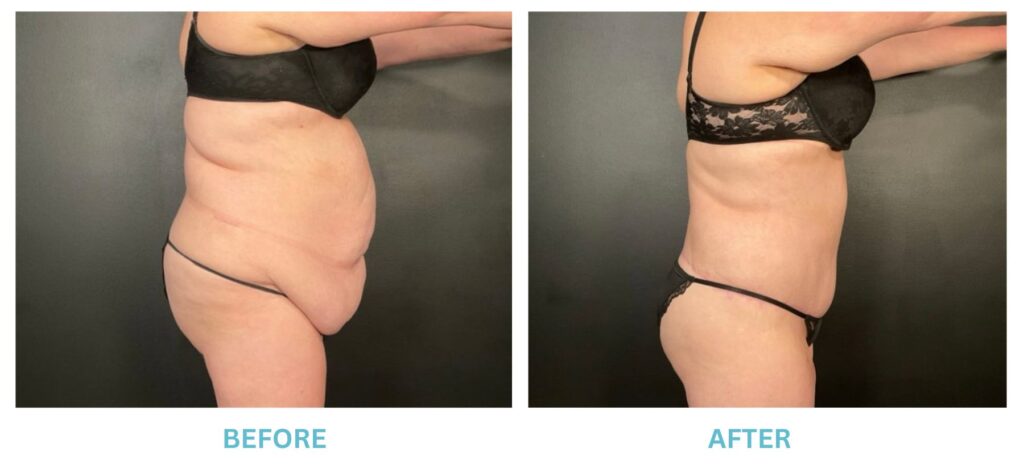
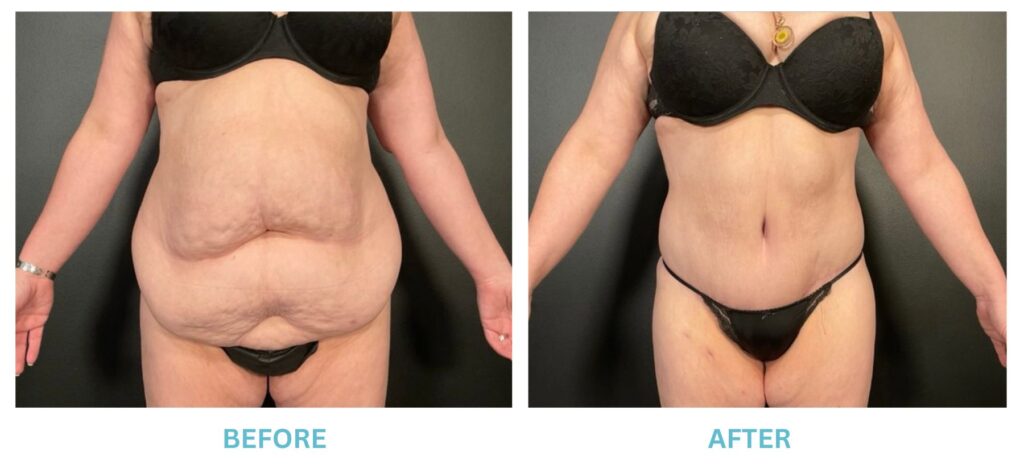
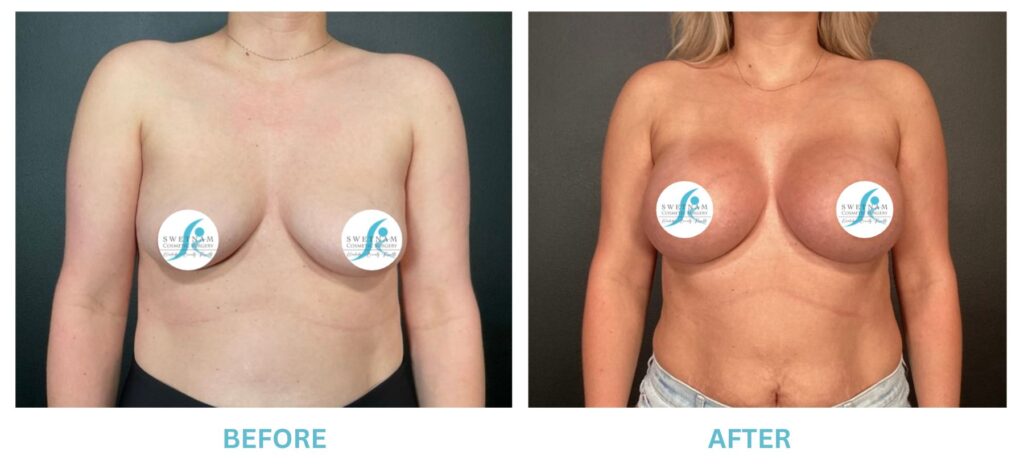
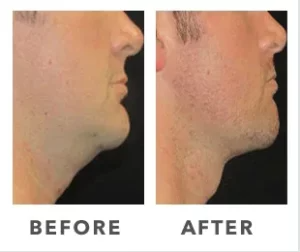
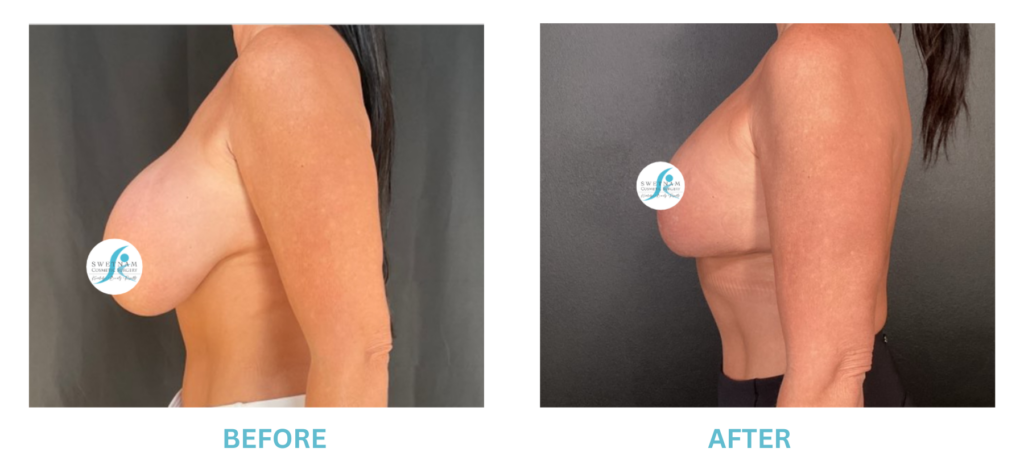
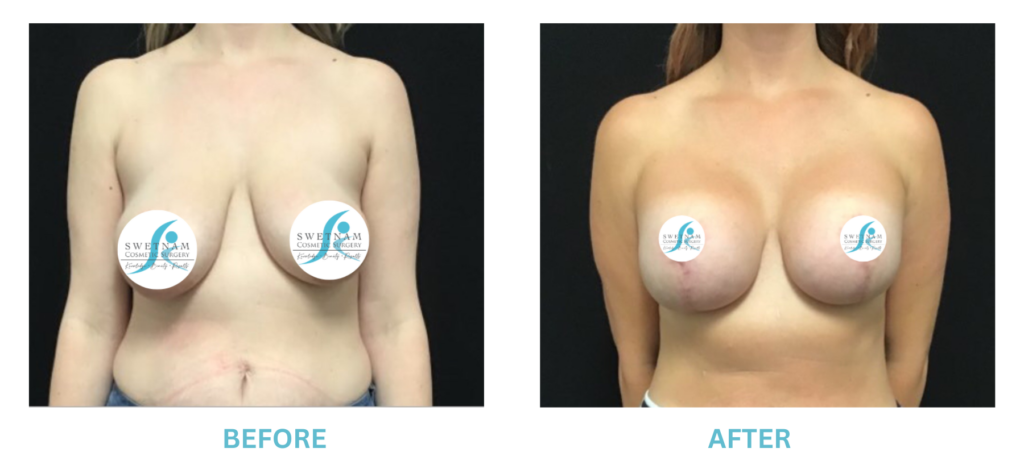
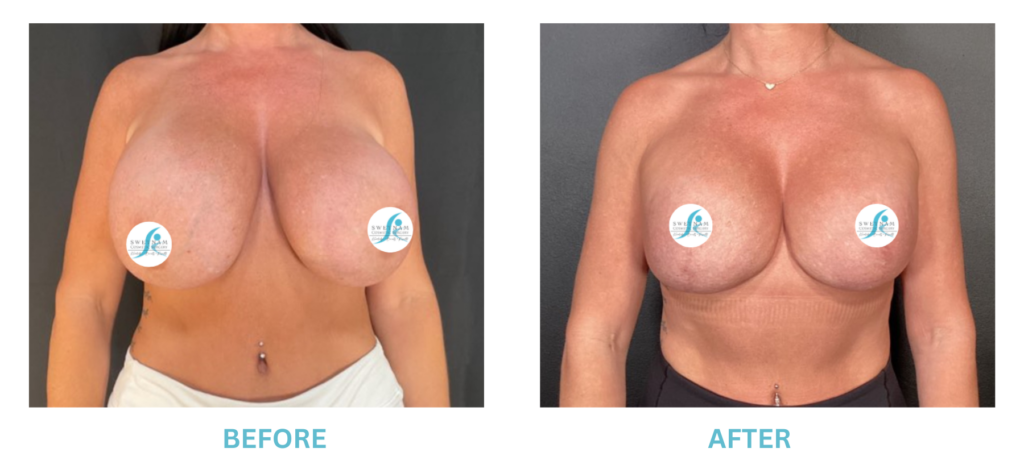
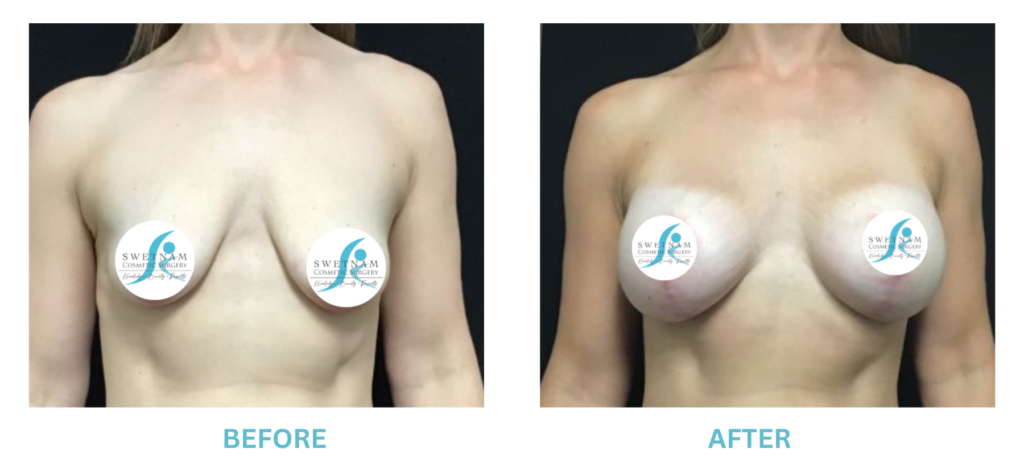
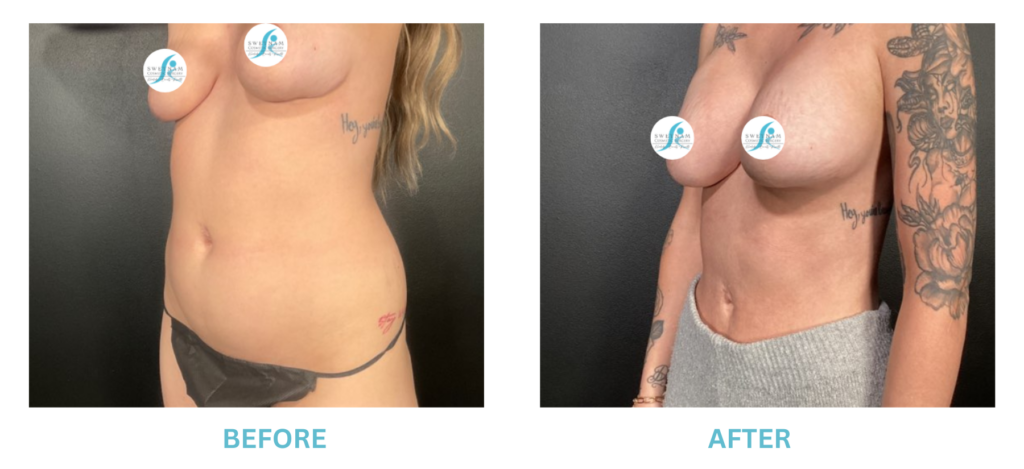
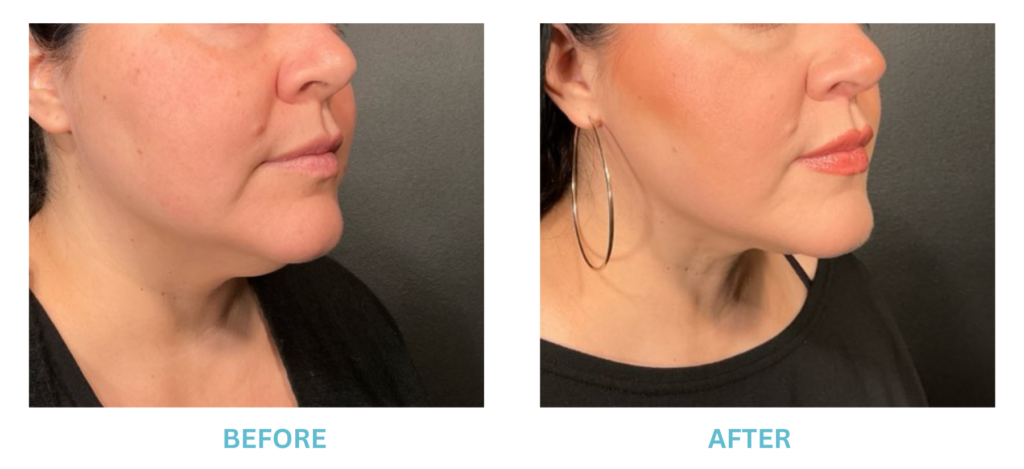
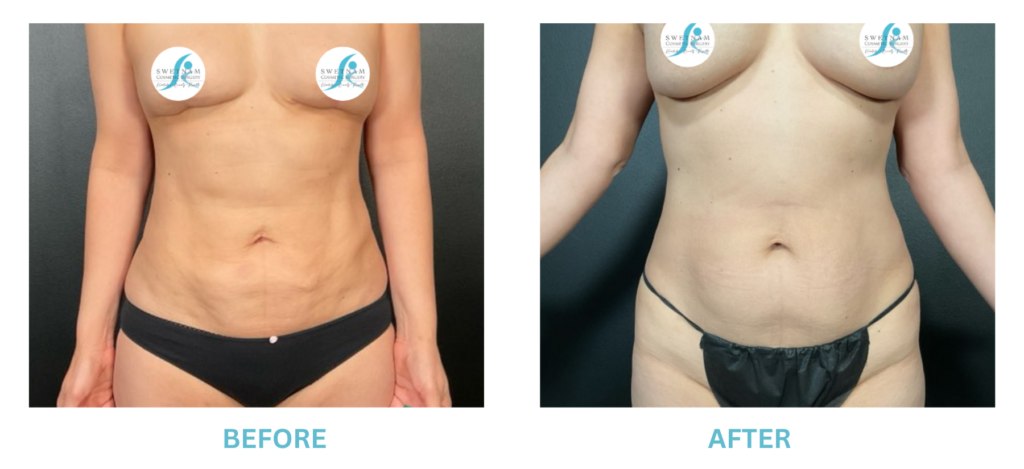
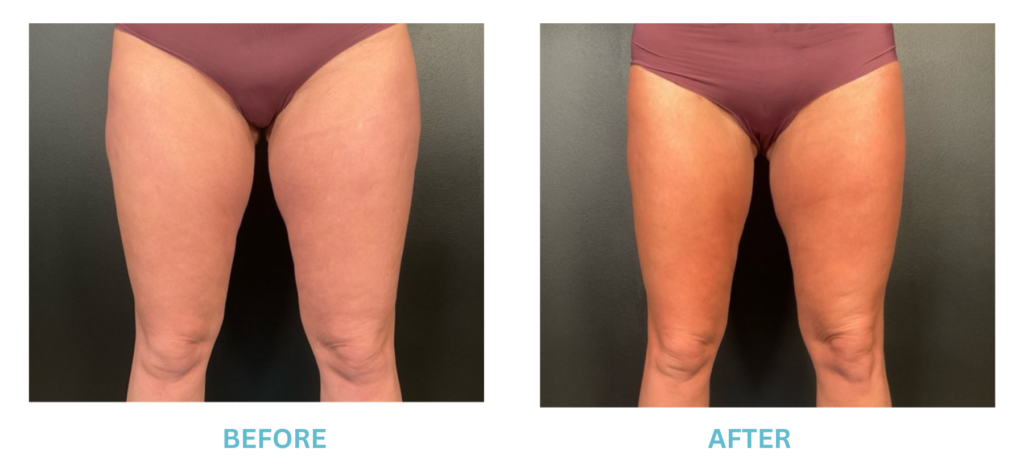
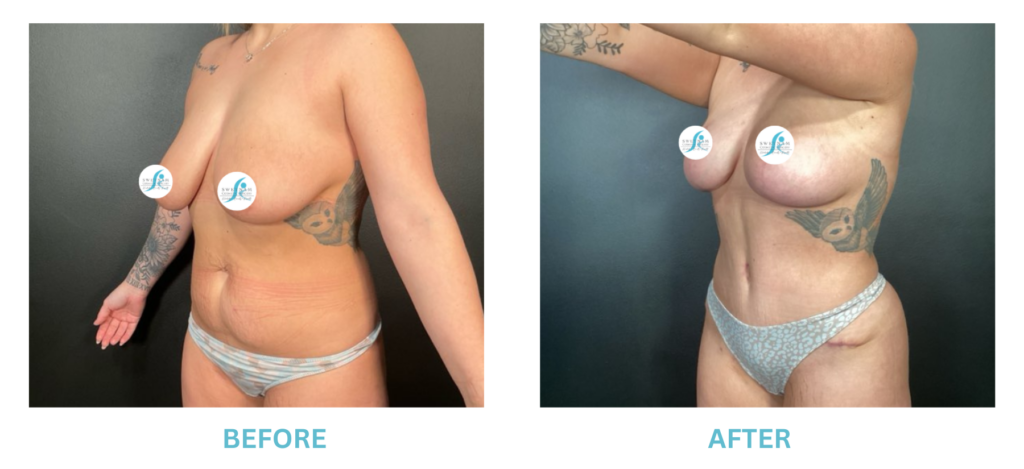
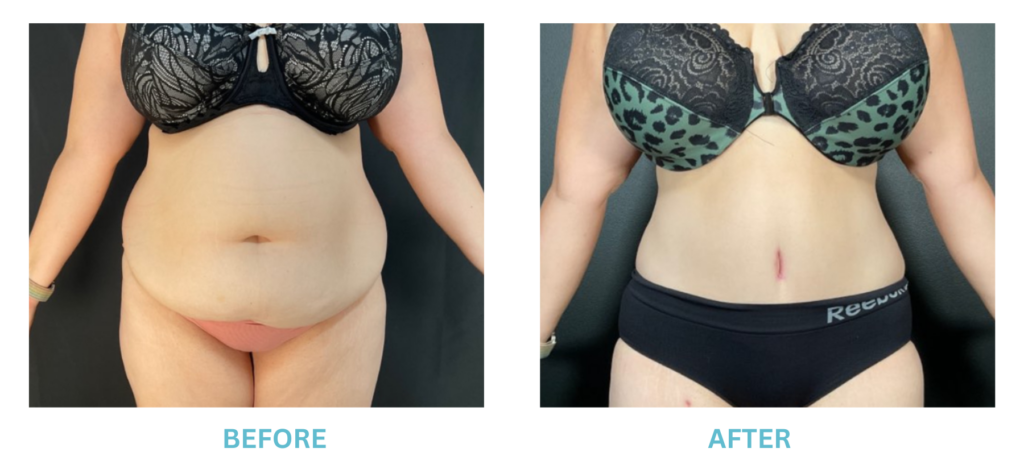
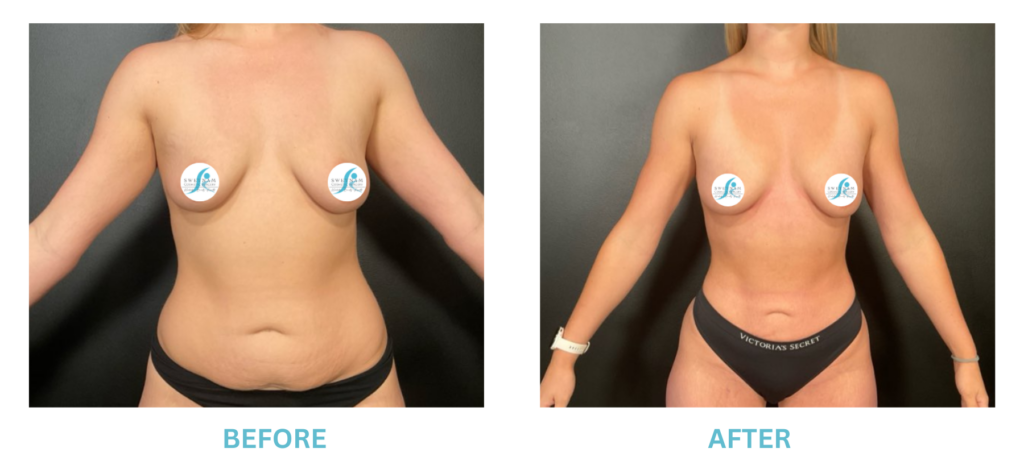
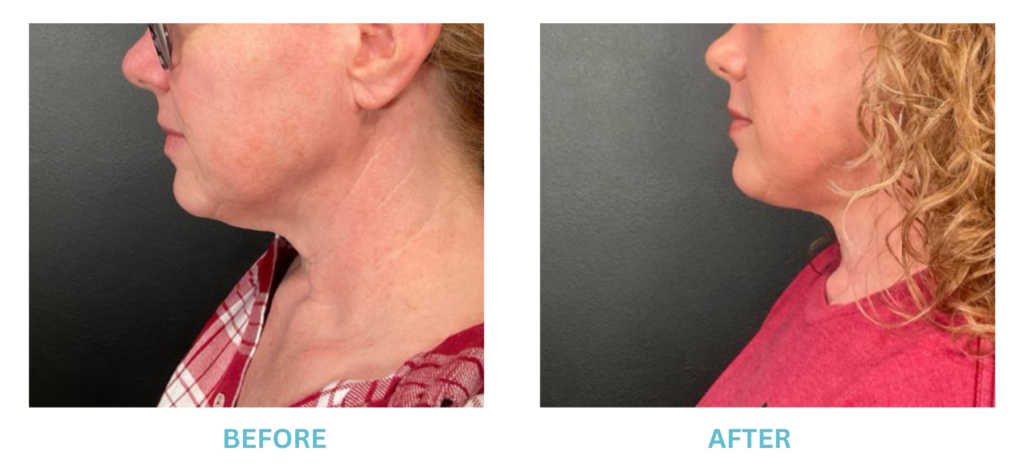
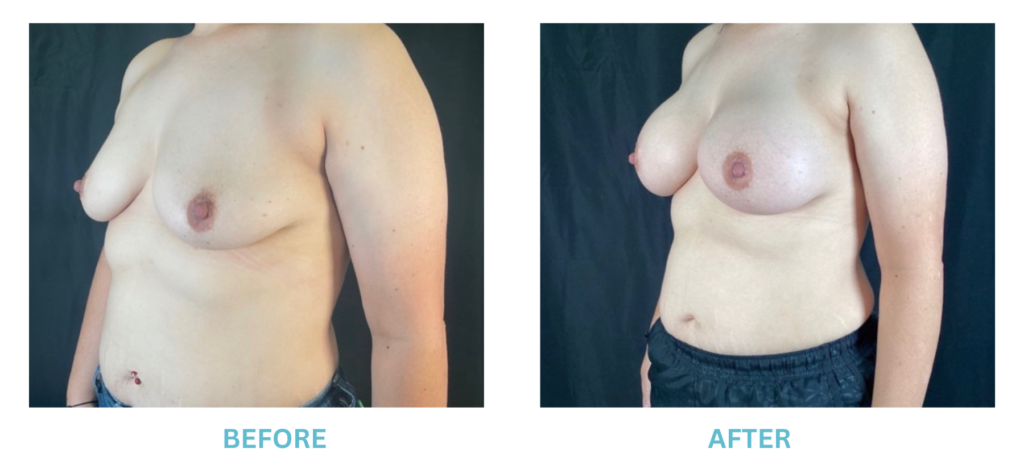
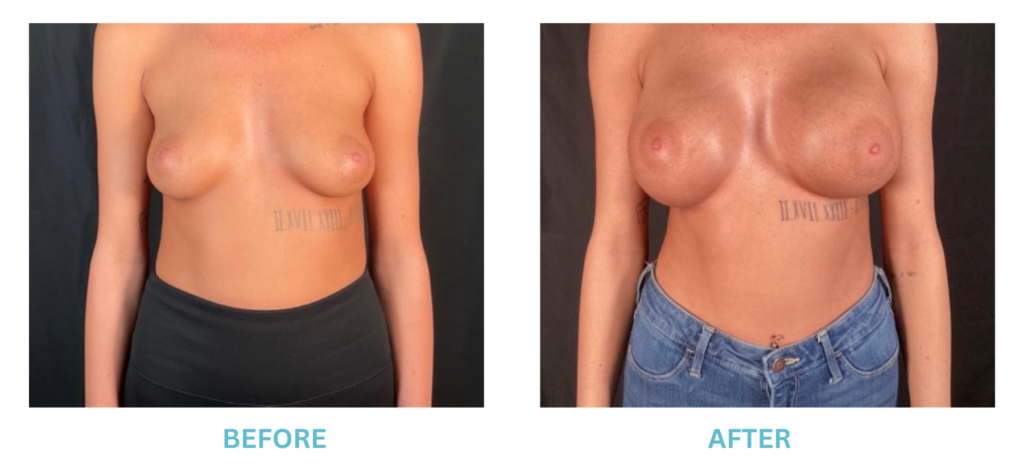
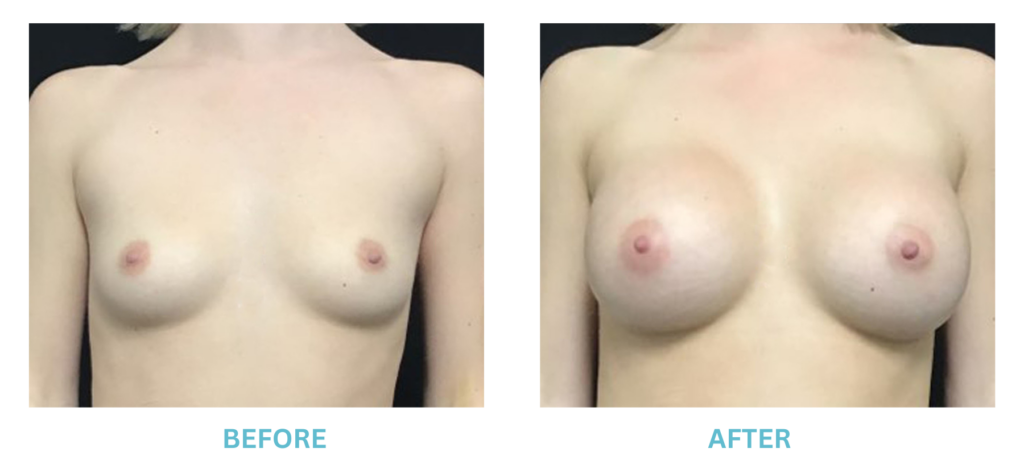
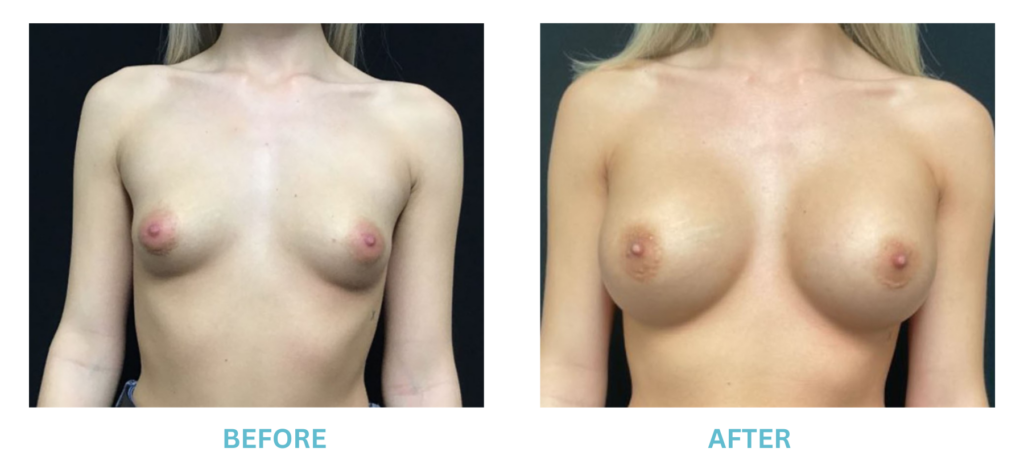
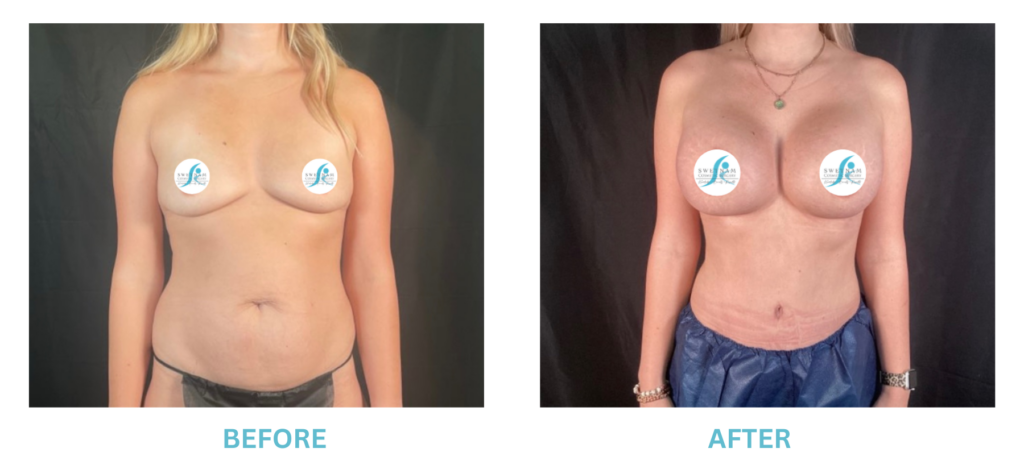
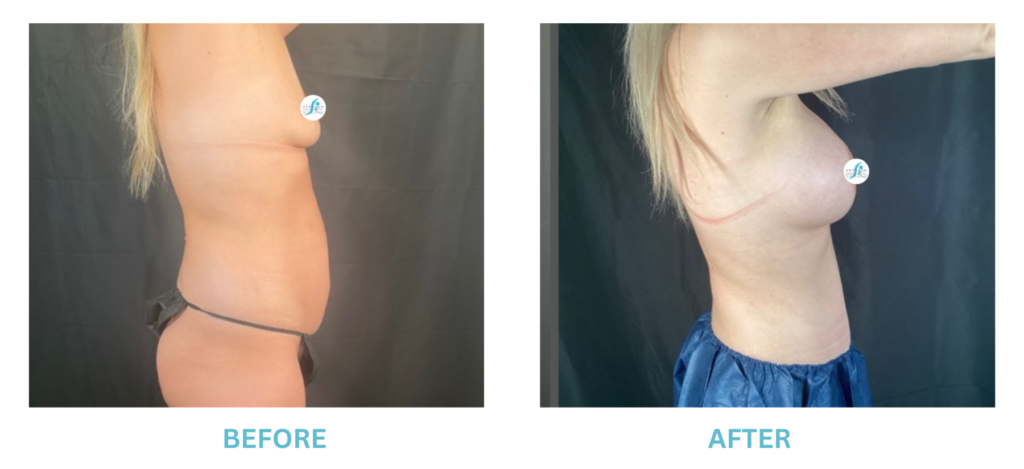
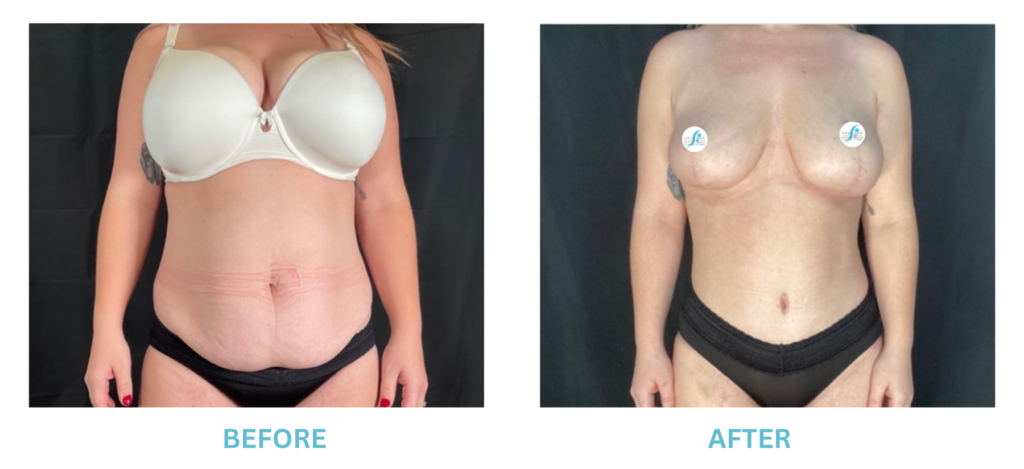
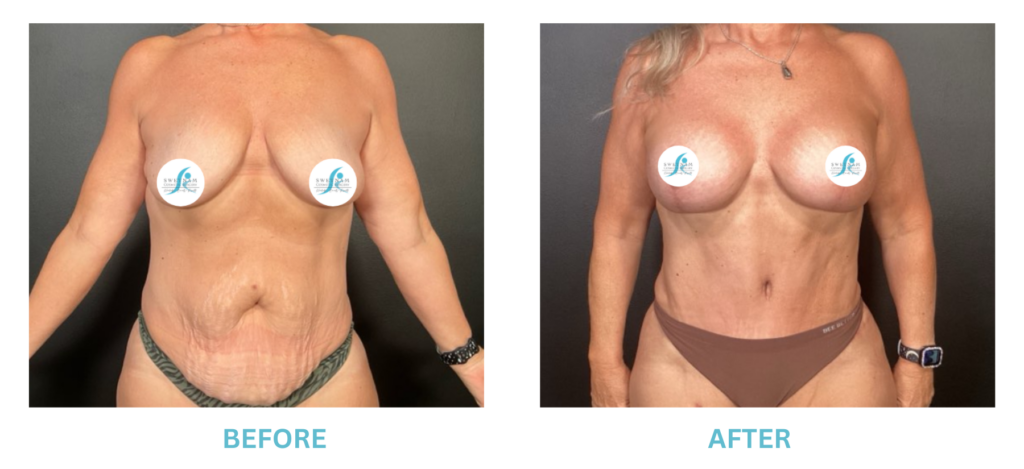
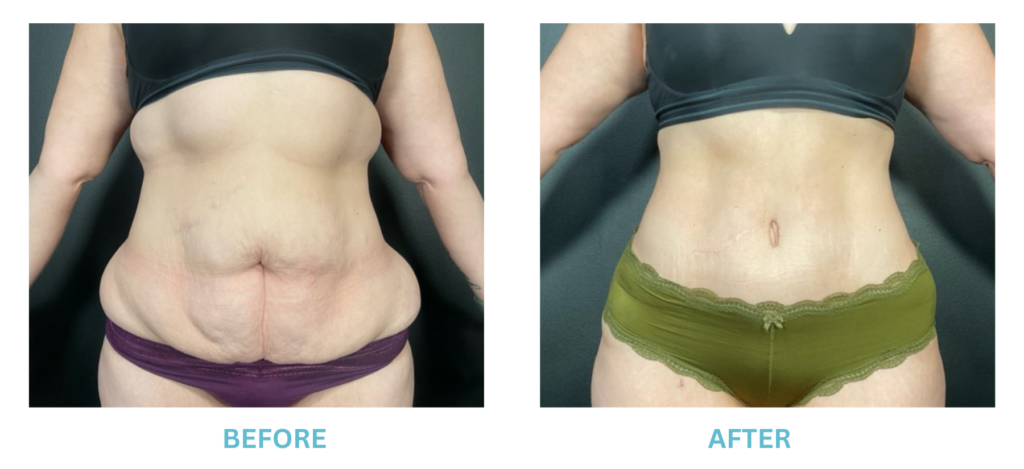
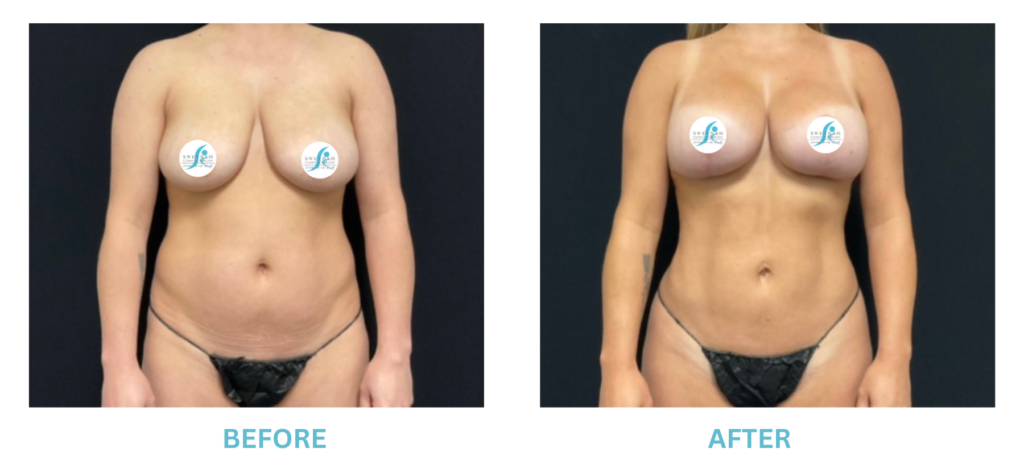
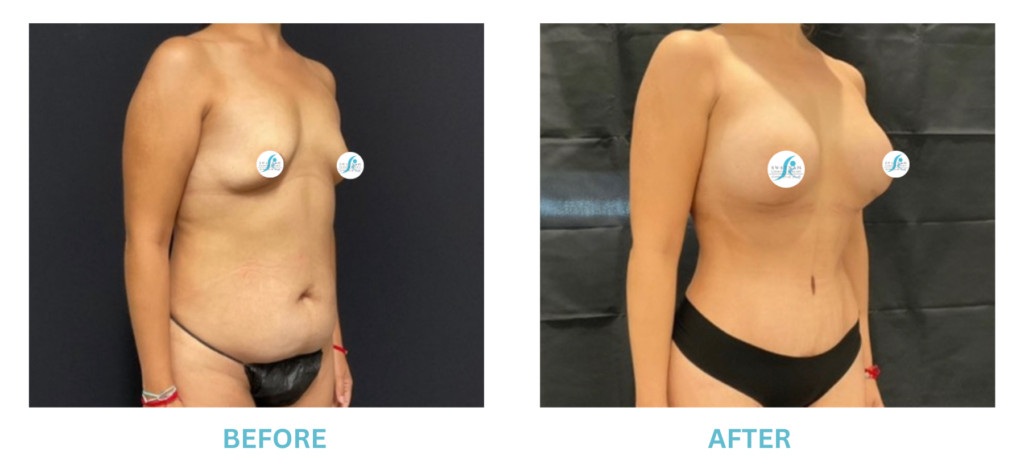
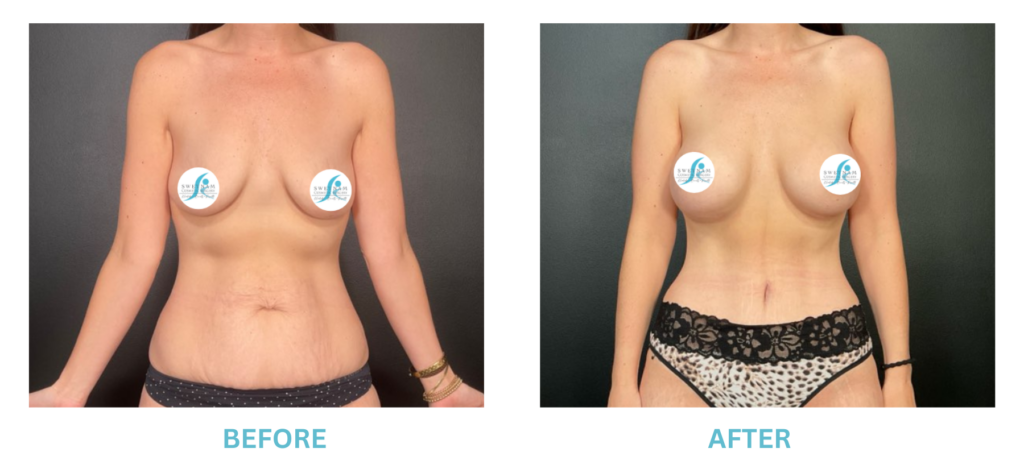
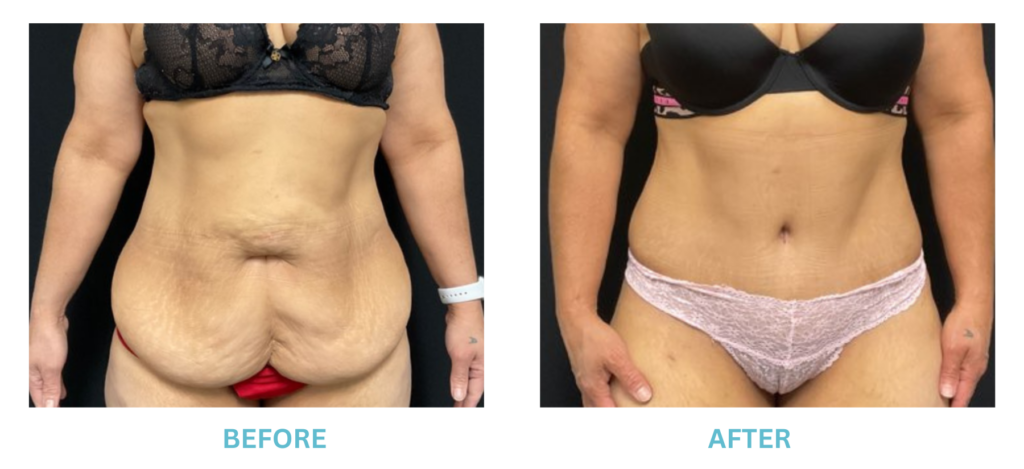
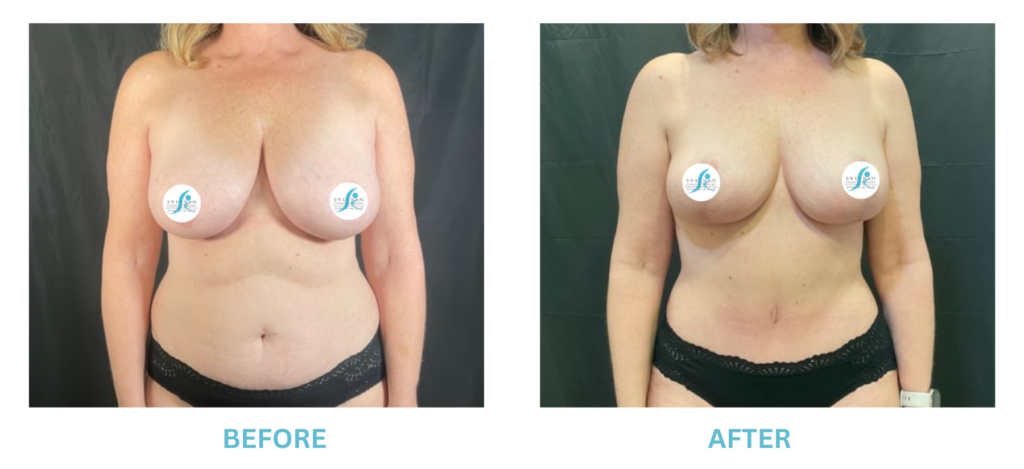
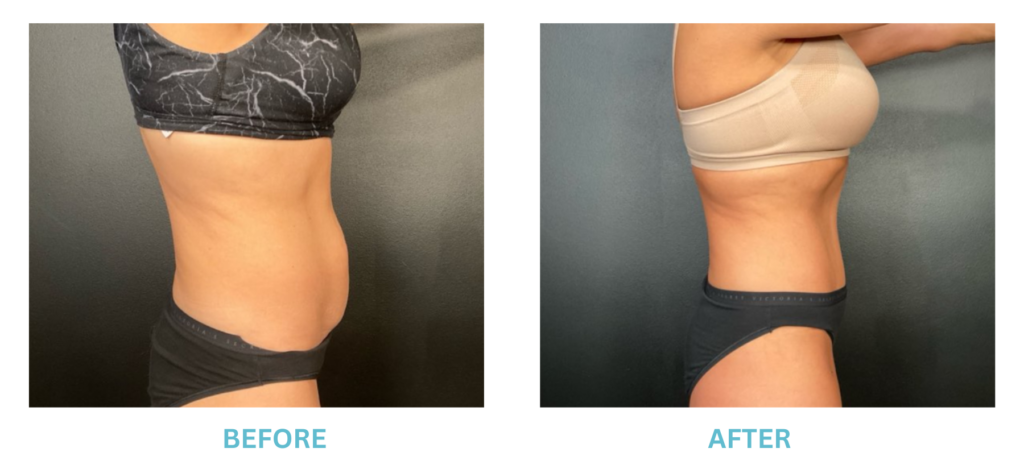
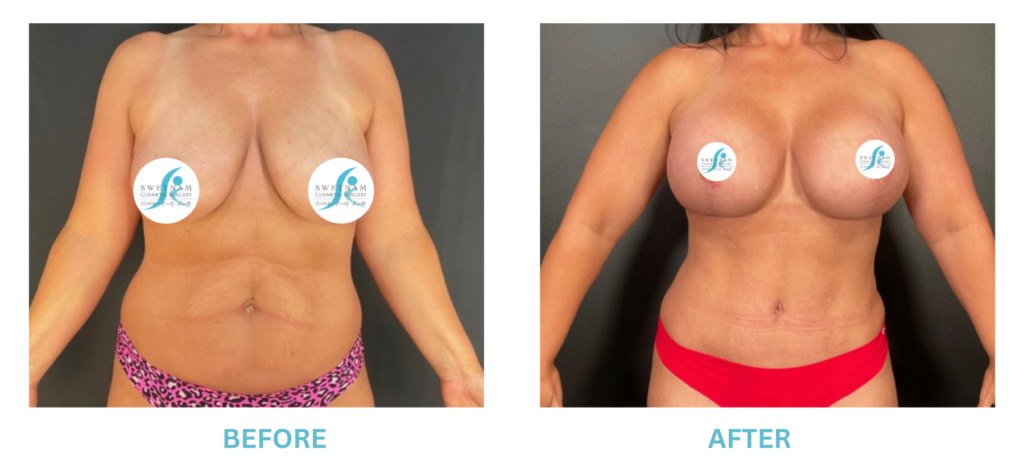
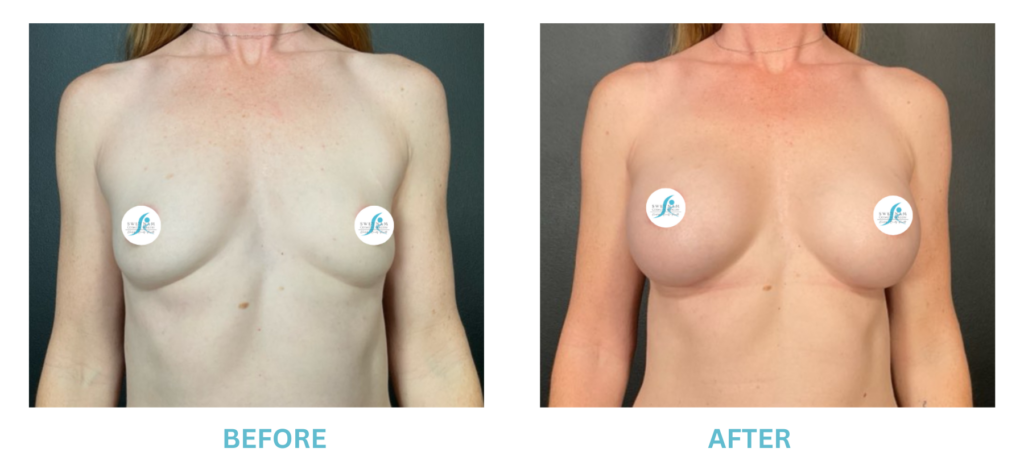
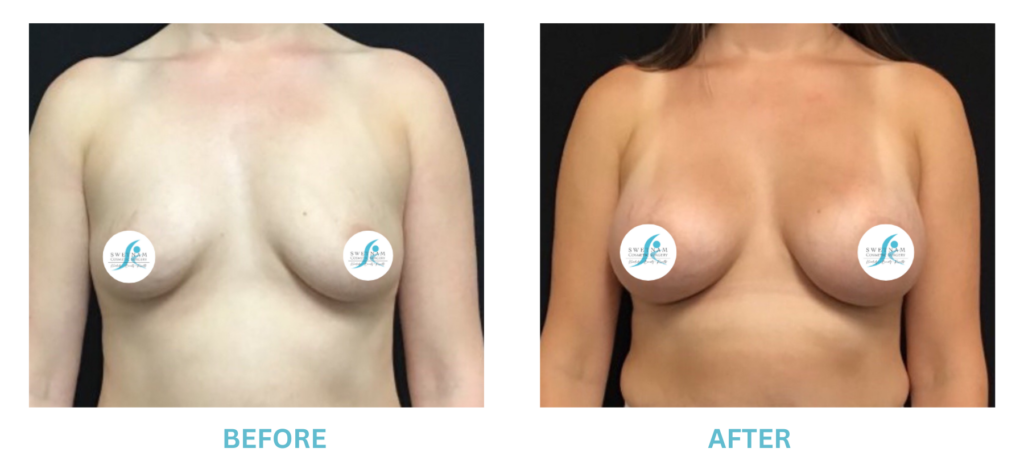
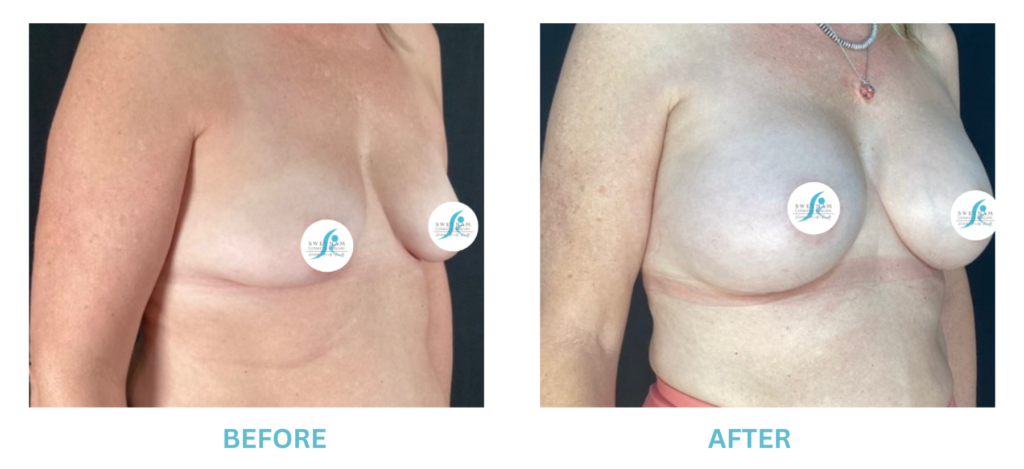
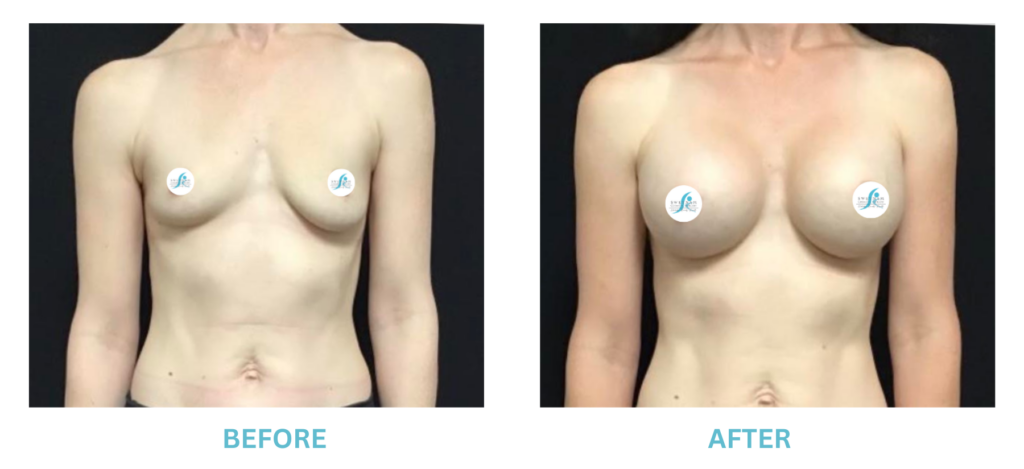
Expanded FDA Breast Implant Regulations and Your Safety

Safety is our highest priority at Swetnam Cosmetic Surgery. For our breast implant patients, expanded safety information and education is now available thanks to updated safety protocols implemented by the US Food and Drug Administration (FDA) in Fall 2021. The goal of the updated regulations is to make sure healthcare providers give clear communication about the possible risk factors associated with breast implants when used for breast augmentation surgery. We support and comply with these requirements when discussing breast implant options with our patients.
The Latest FDA Breast Implant Regulations
Responding to patient reports of complications following breast implant surgery, the FDA gathered data over a 2 year period to determine the incidence of 2 specific potential risks: anaplastic large cell lymphoma (BIA-ALCL) and breast implant illness (BII). While the causes of these complications continue to be explored, the FDA found that they are prevalent enough to necessitate increased safety information for prospective breast implant patients.
To help patients receive complete risk information before making an informed decision about surgery, the updated FDA requirements expand labeling for all legally marketed breast implants. Patients now receive the following before their breast augmentation surgery:
- Boxed warning – This warning appears on the box containing each breast implant, as delivered by the manufacturer.
- Patient decision checklist – We review this checklist with the patient prior to surgery so that known risks are fully discussed. Both the patient and Dr. Swetnam must sign off on the checklist before surgery.
- Silicone gel rupture screening recommendations – Because silicone gel implants can rupture without any obvious physical symptoms, patients are provided with updated screening recommendations to ensure their implants remain in good shape.
- Breast implant description – In essence this is an “ingredient label” for each implant, listing materials used in the manufacturing process.
- Patient device card – Patients are provided with this card so that they have an overview of key information about their specific implant.
Standardizing Safety Information
Prior to surgery, patients have always received information about known breast implant surgery risks. The updated FDA regulations were put in place to standardize the information patients receive, and expand information about both BIA-ALCL and BII. Though the risk of a breast implant patient developing BIA-ALCL is low (currently estimated at about 1 in 450 patients with textured breast implants), BII appears to be more common, though its symptoms seem to vary widely and some medical providers question the validity of the diagnosis.
What Does This Mean for Your Breast Augmentation?
It is important for people considering breast implants to remember that the updated FDA guidelines do not mean that breast augmentation surgery has become less safe, or that breast implants carry serious risks. In fact, breast implants are one of the most studied medical devices of all time, and hundreds of thousands of them are implanted safely each year.
We support our patients making an informed choice about surgery after understanding all of the upsides and potential risks. By expanding and standardizing implant safety information, the FDA has helped us provide our patients with the information they need when considering their options. If you are thinking about breast augmentation, we encourage you to schedule a consultation so we can answer all your questions.