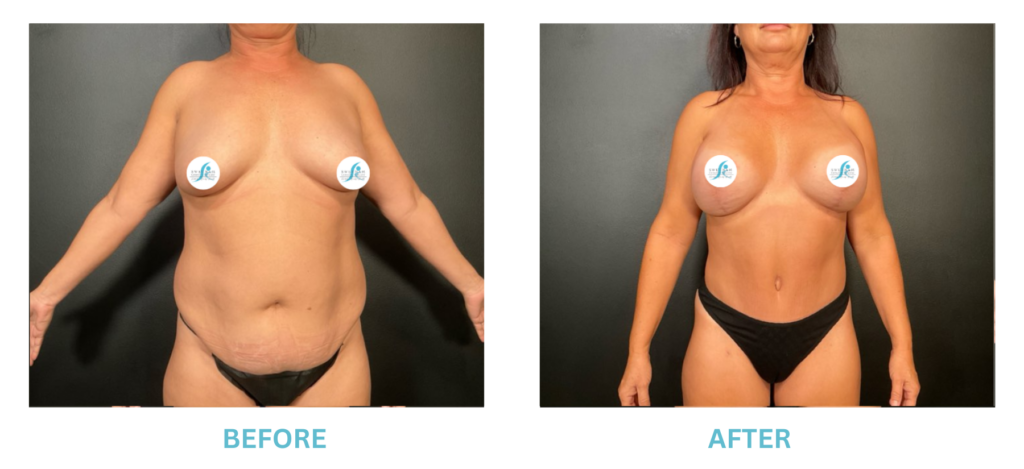
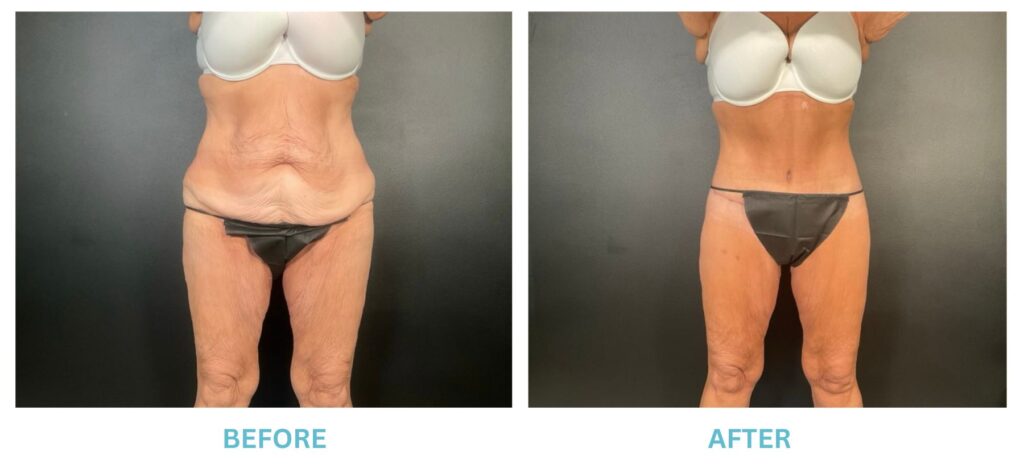
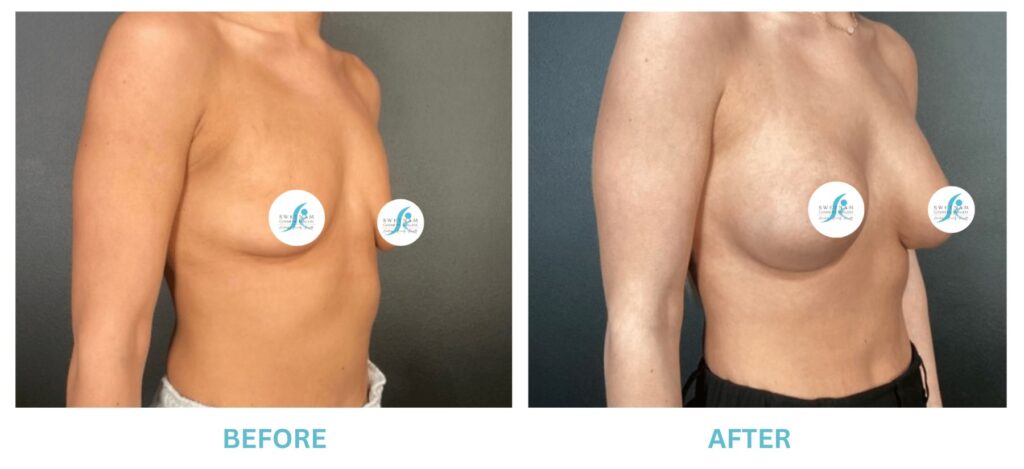
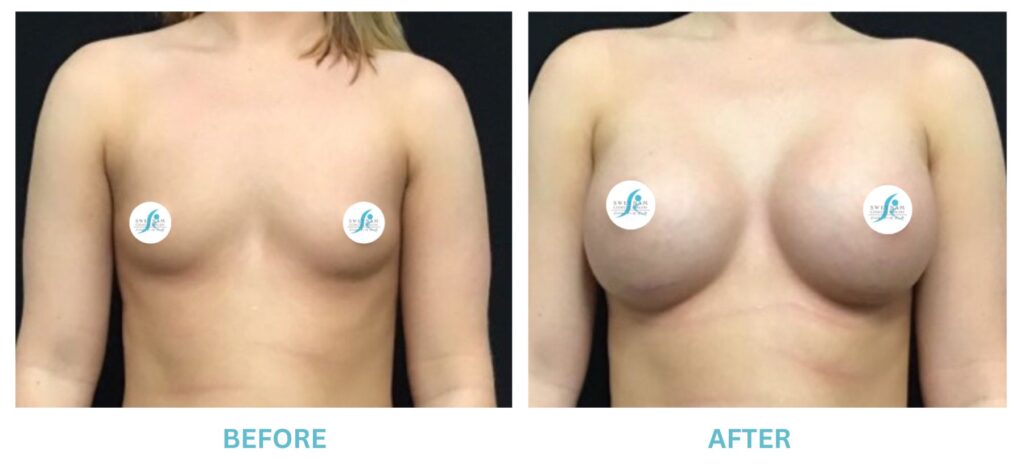
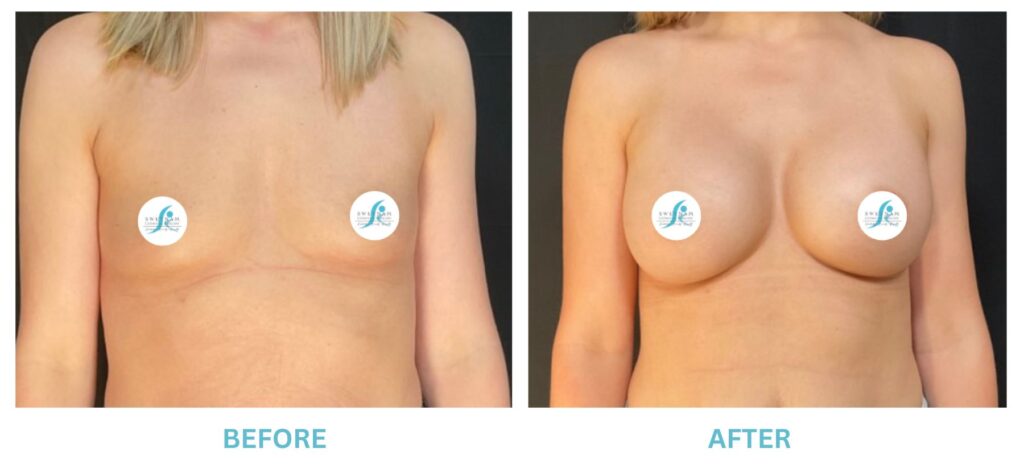
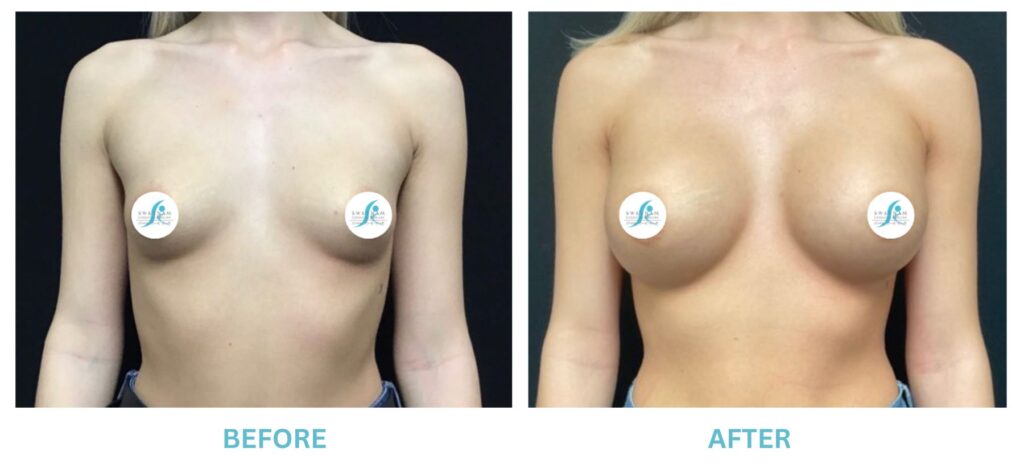
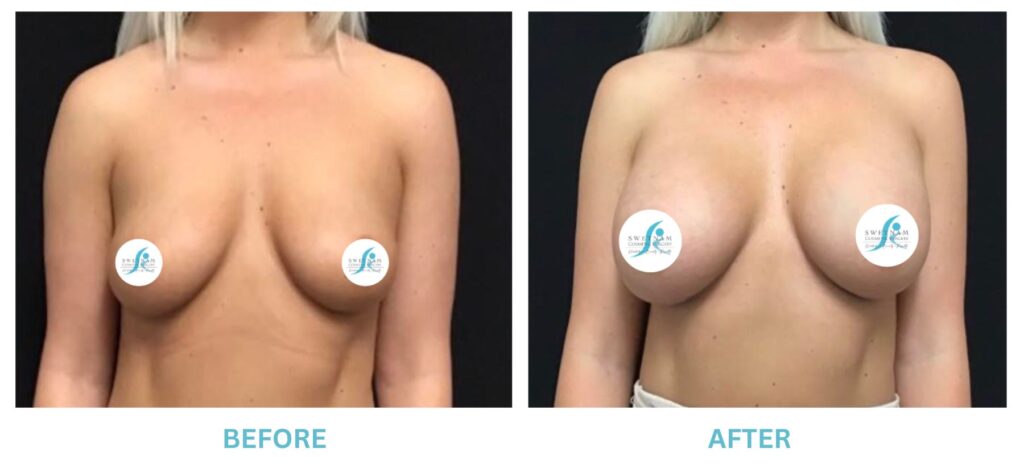
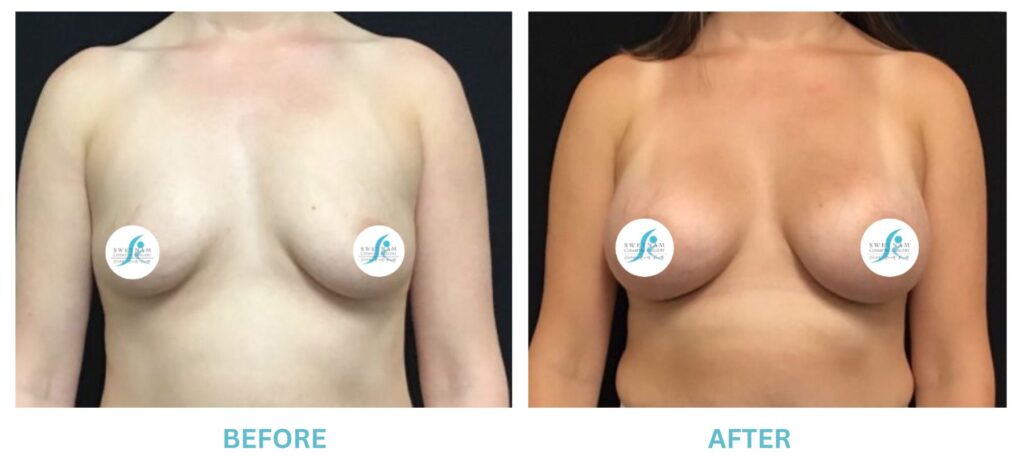
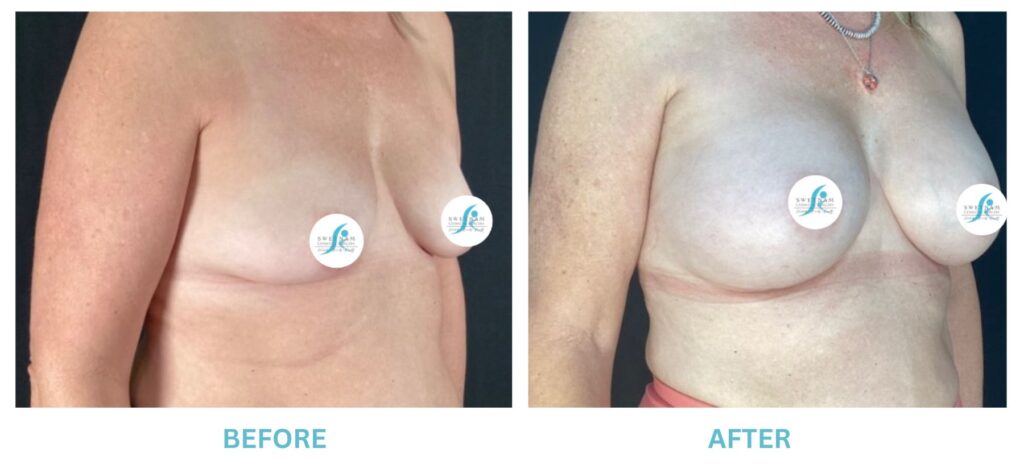
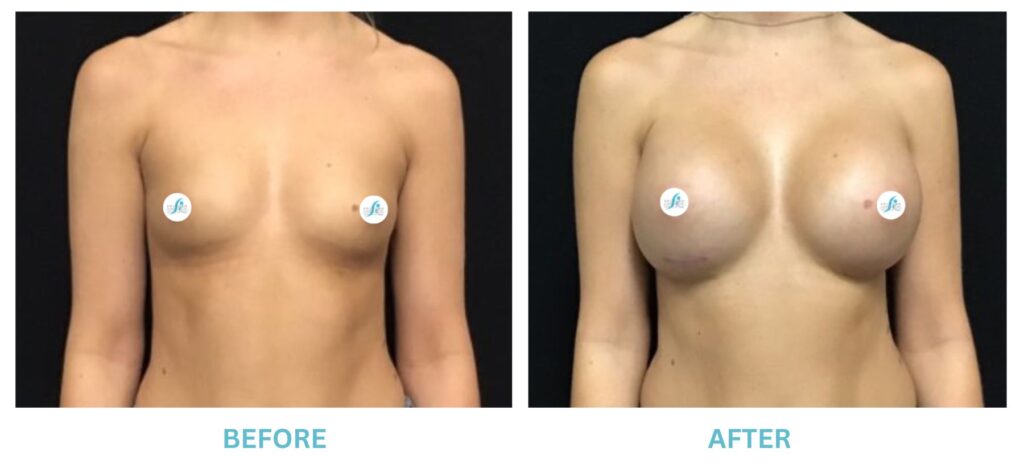
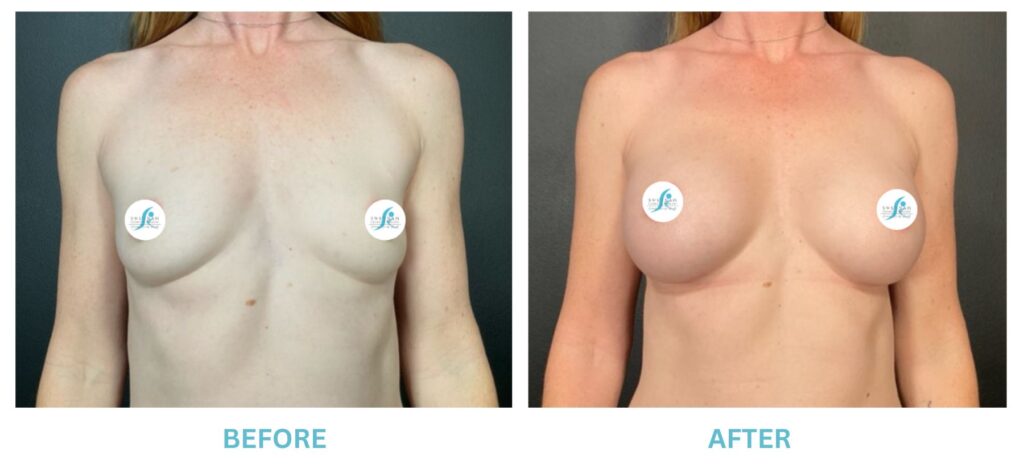
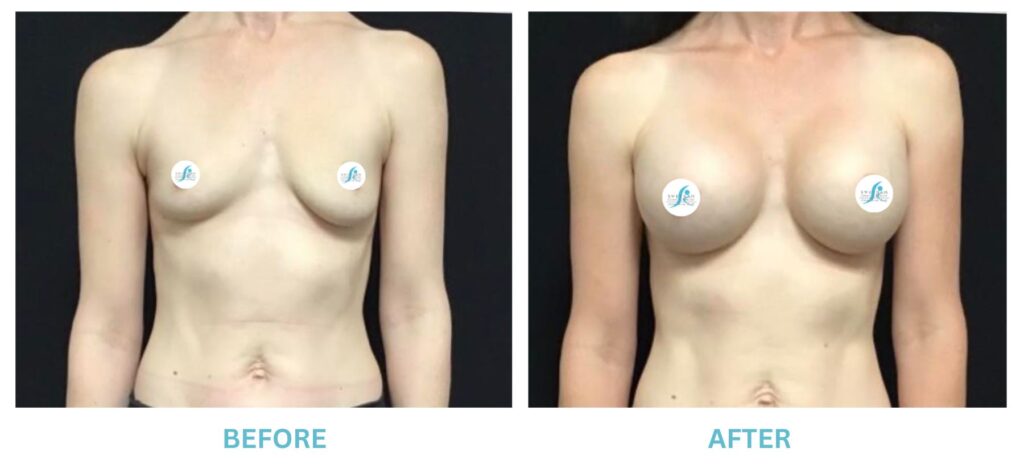
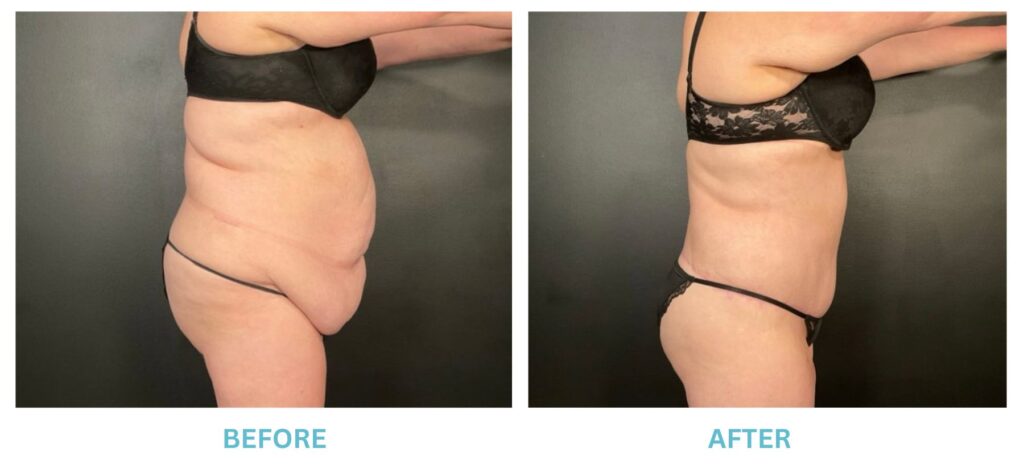
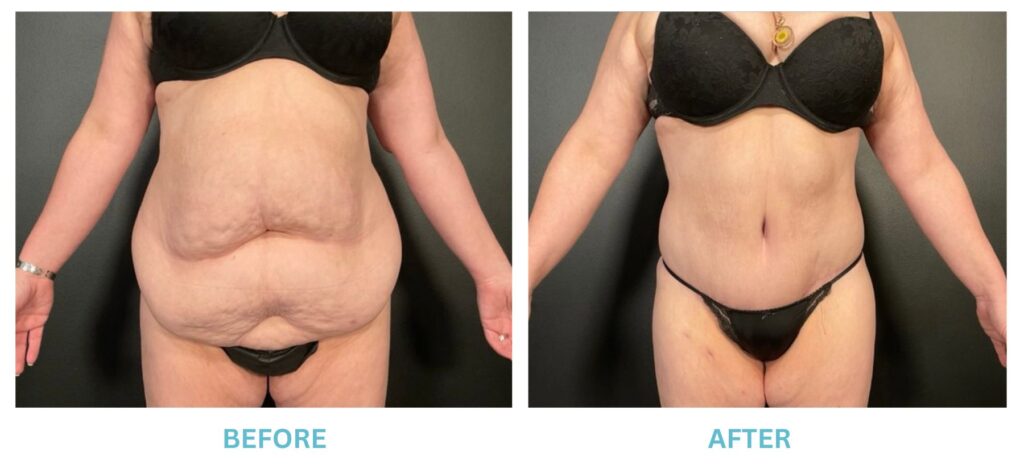
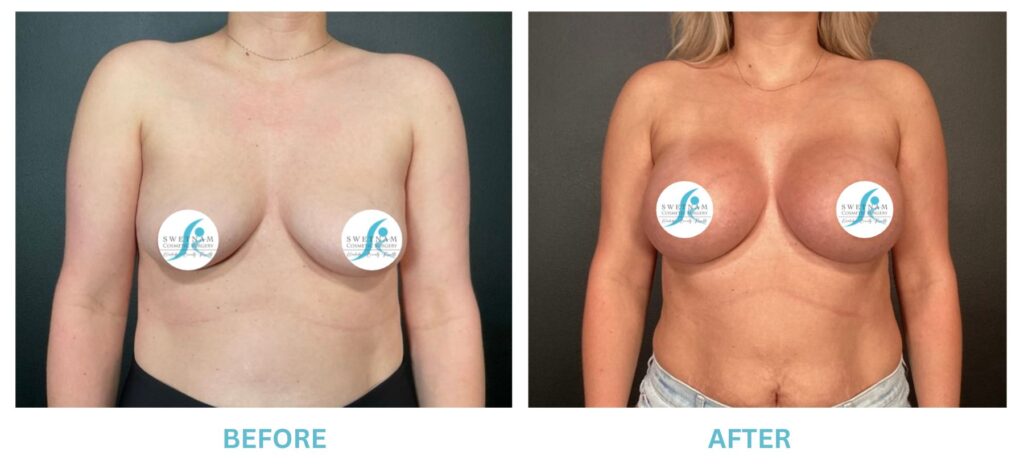
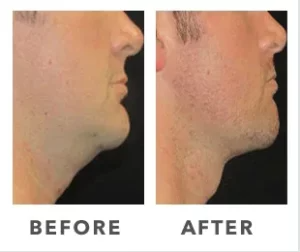
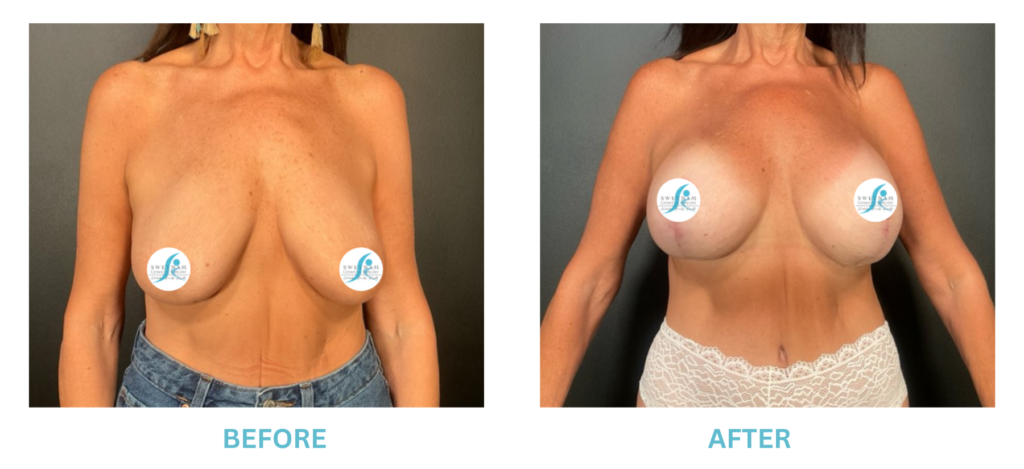
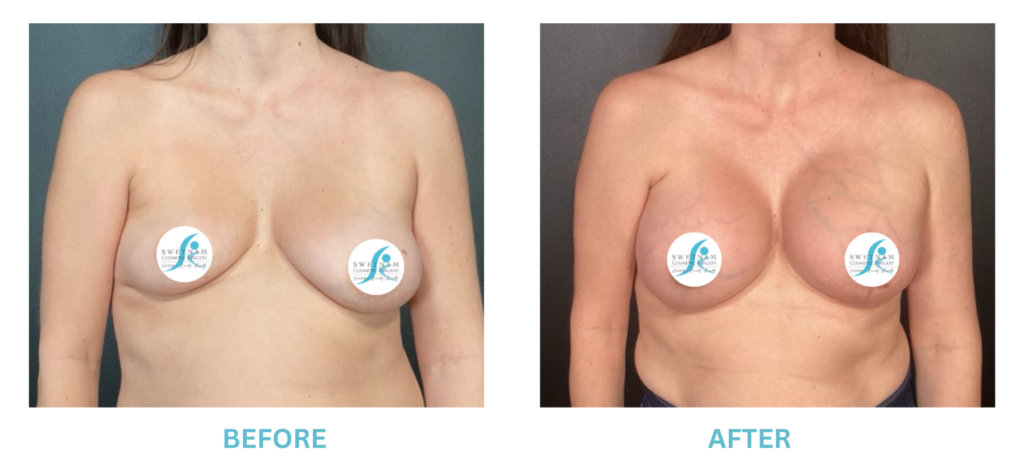
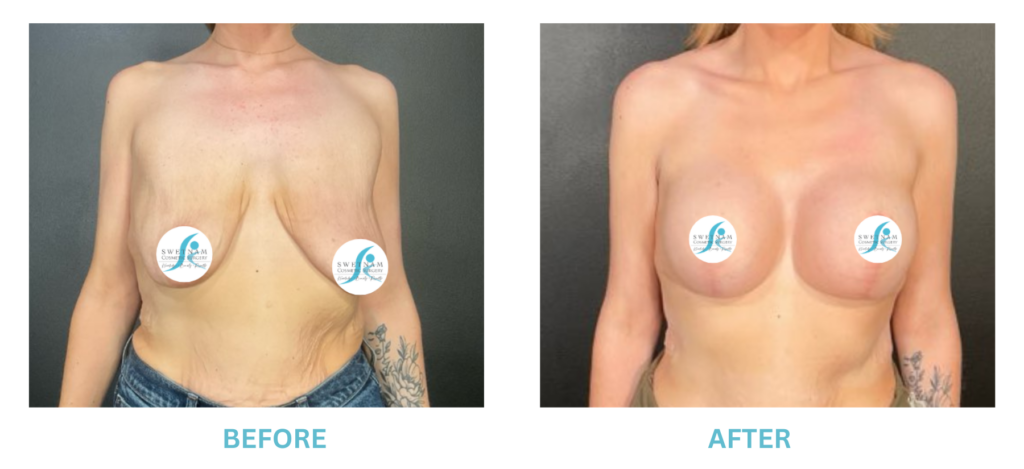
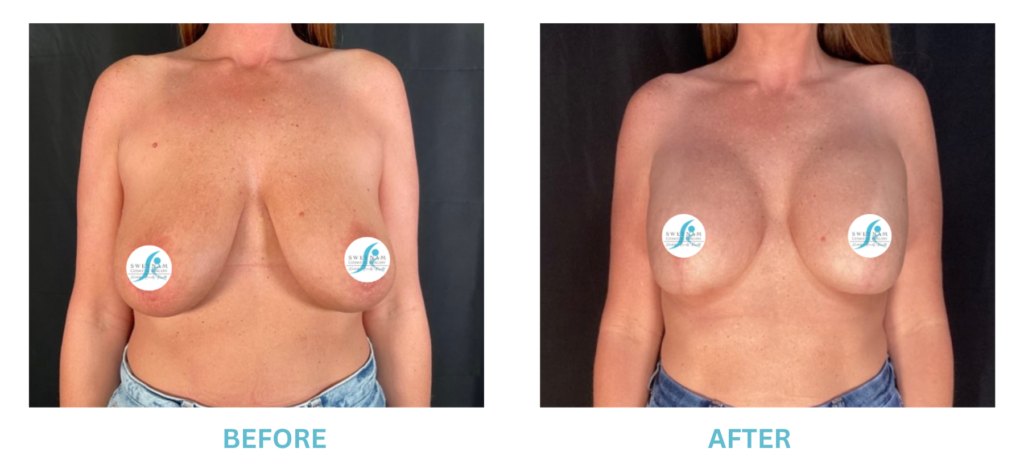
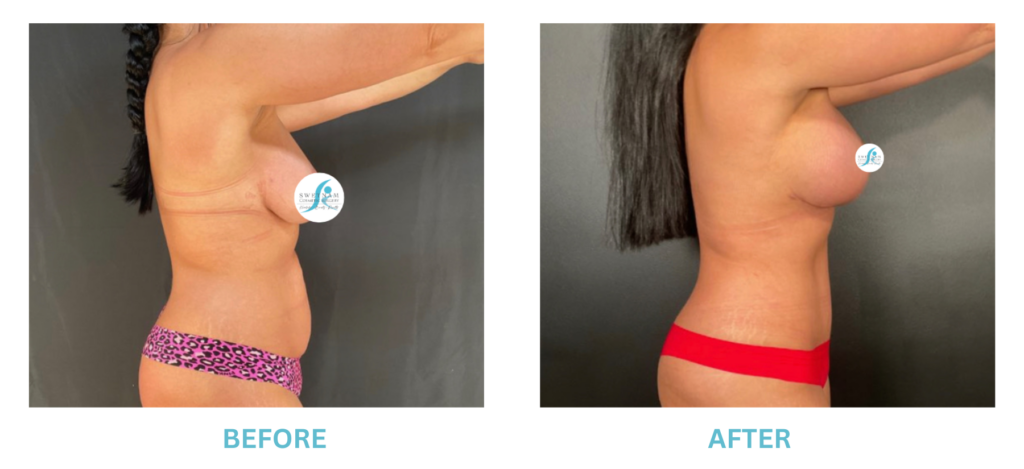
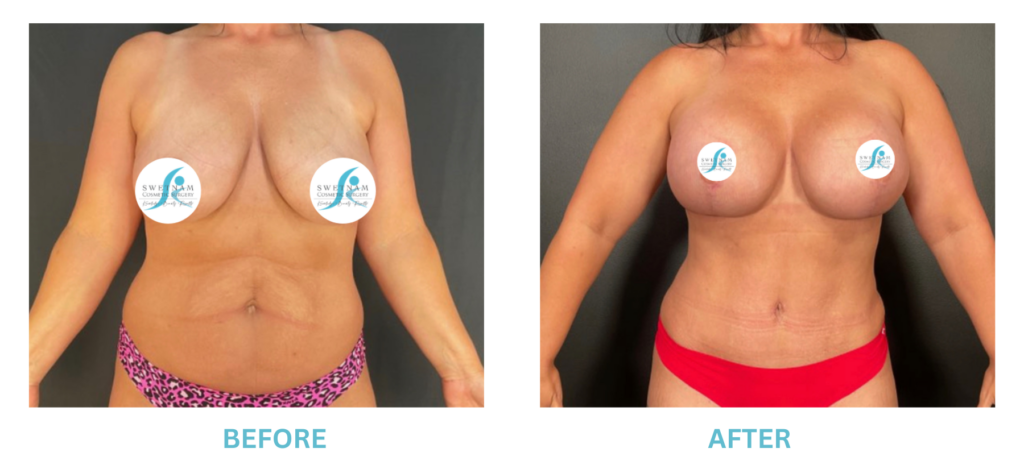
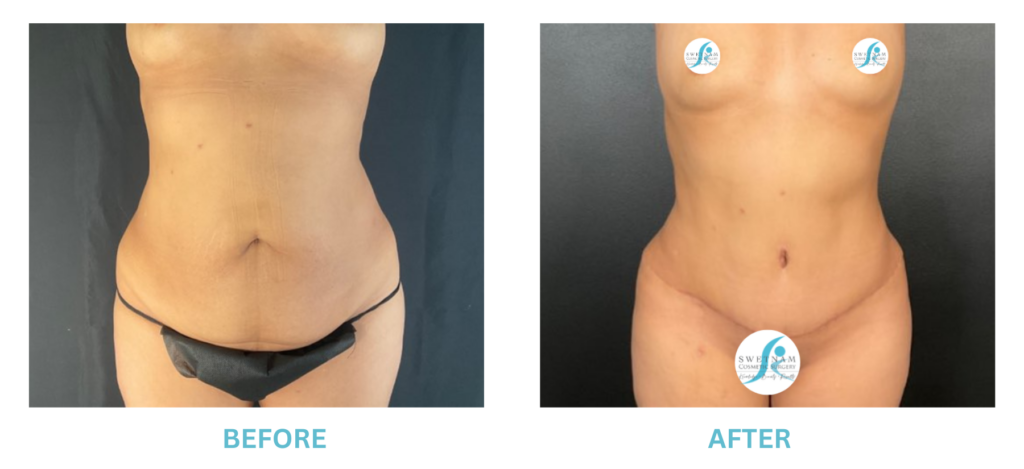
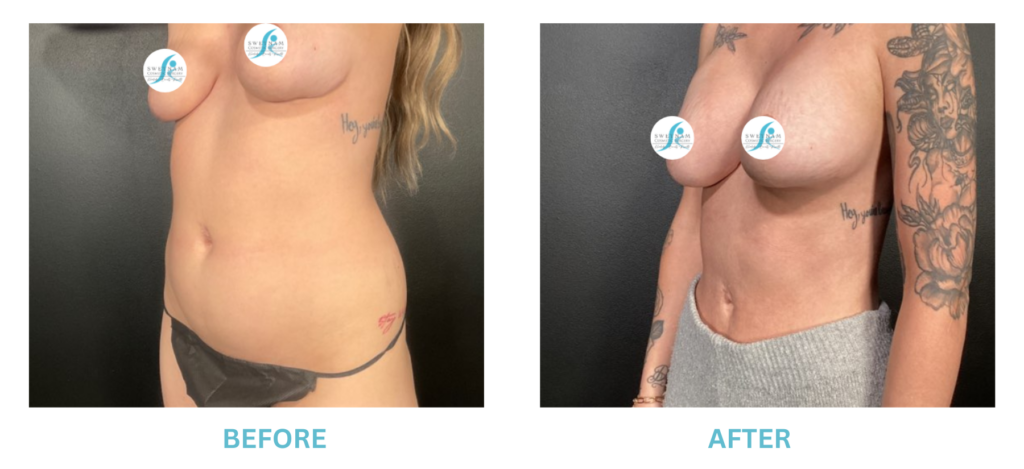
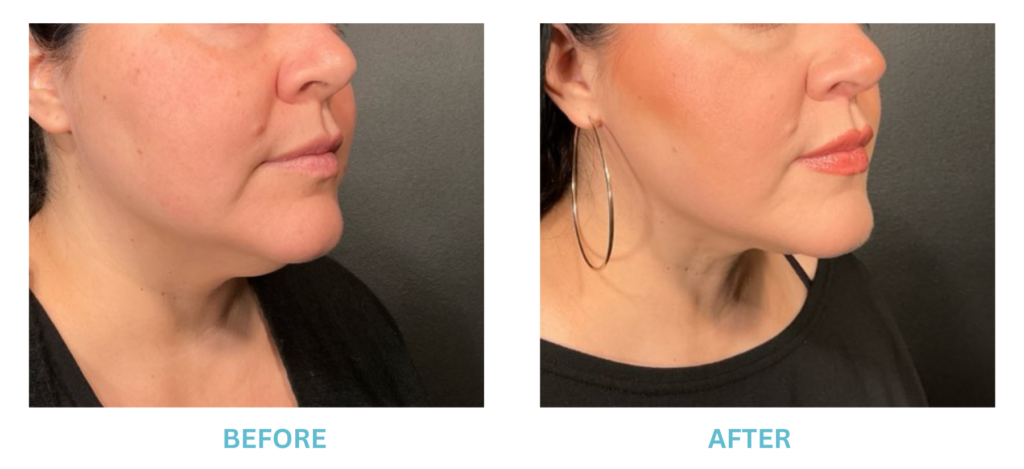
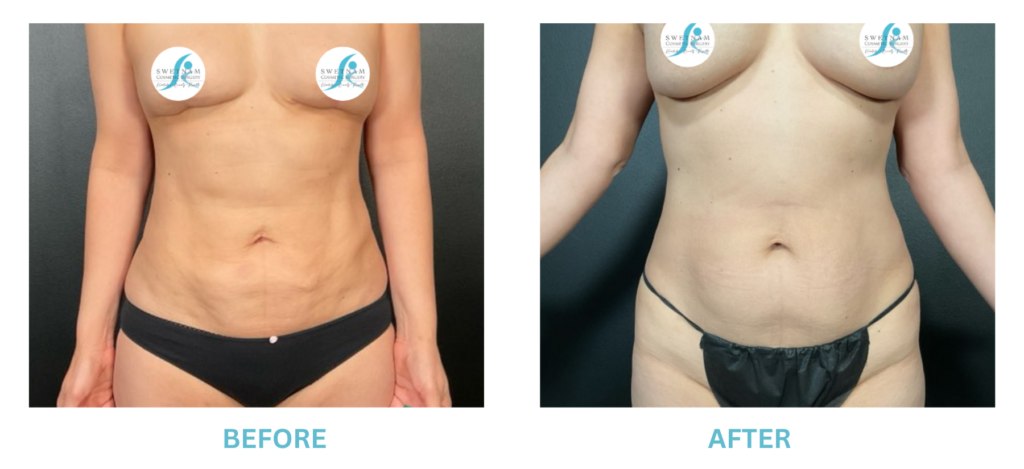
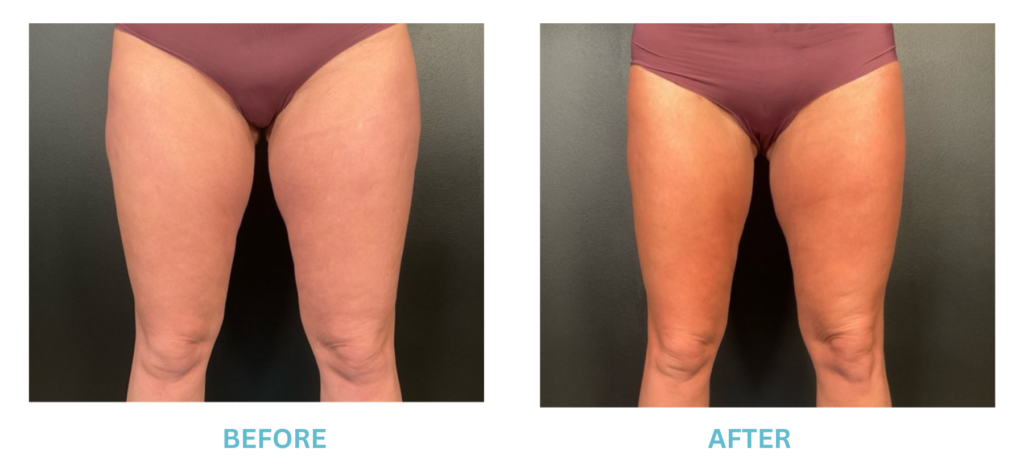
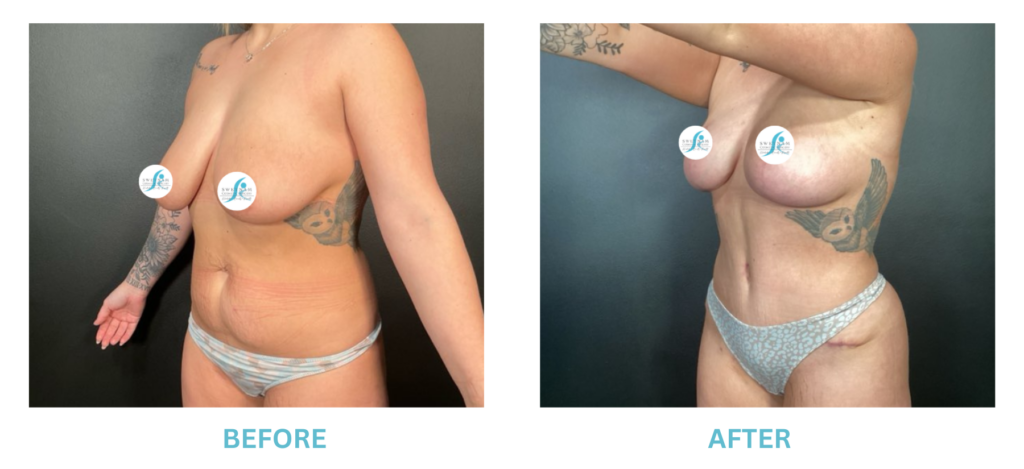
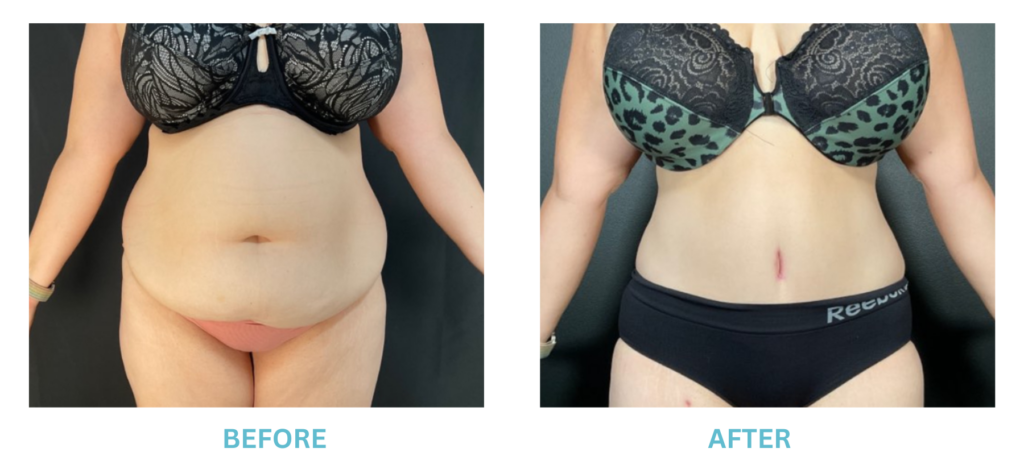
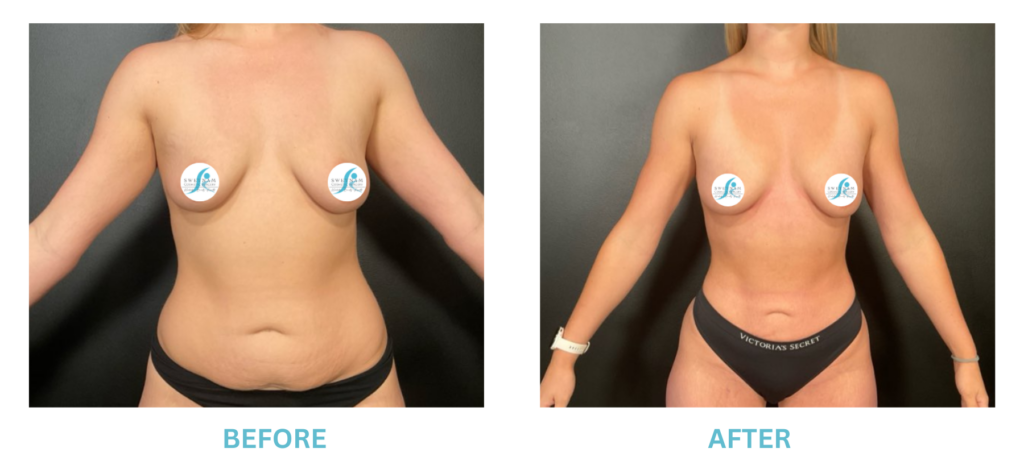
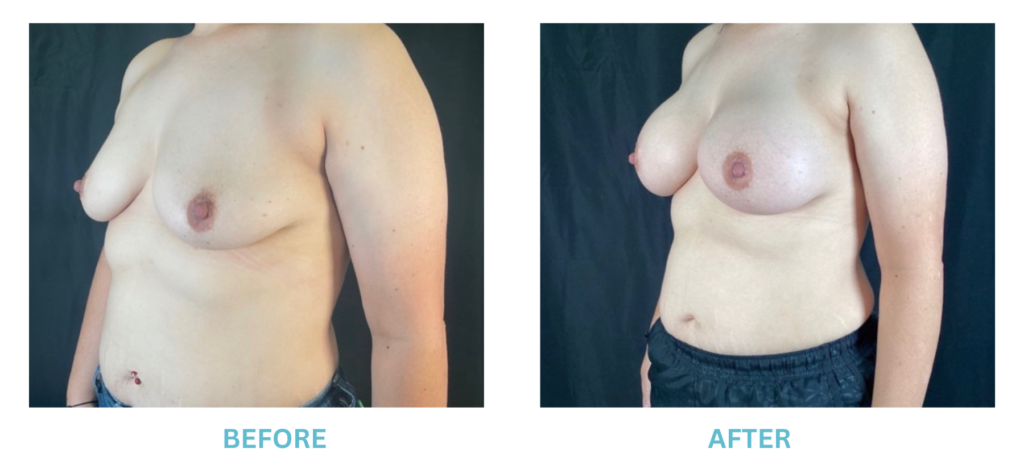
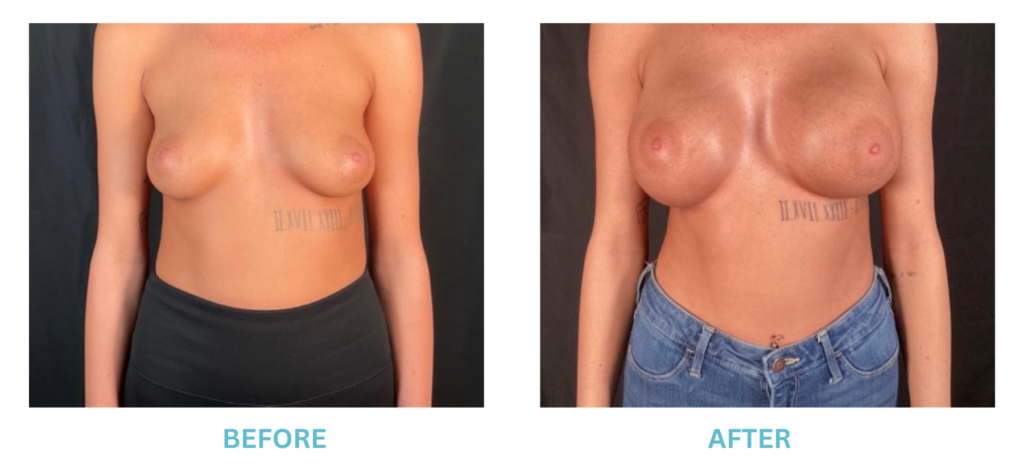
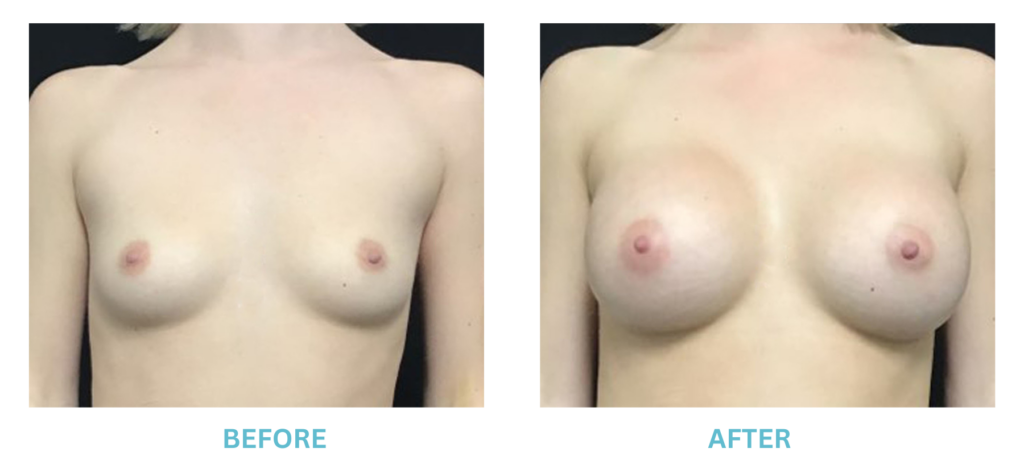
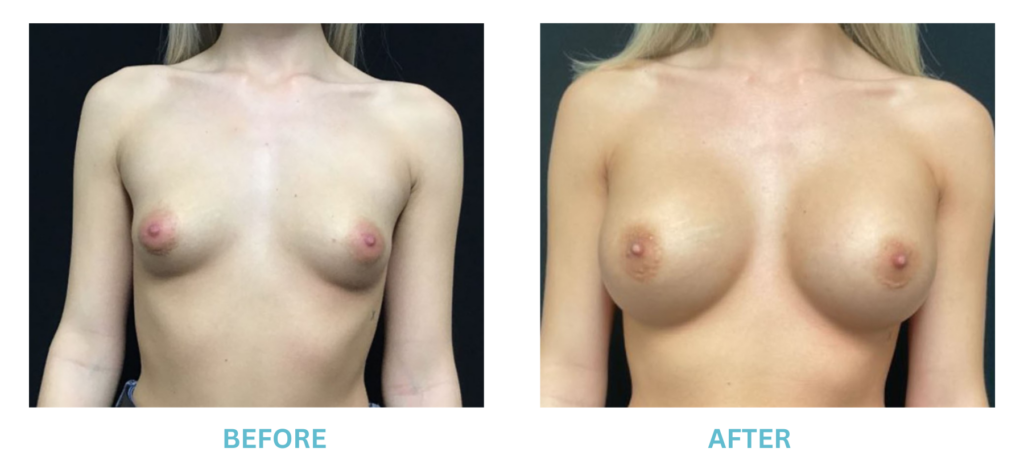
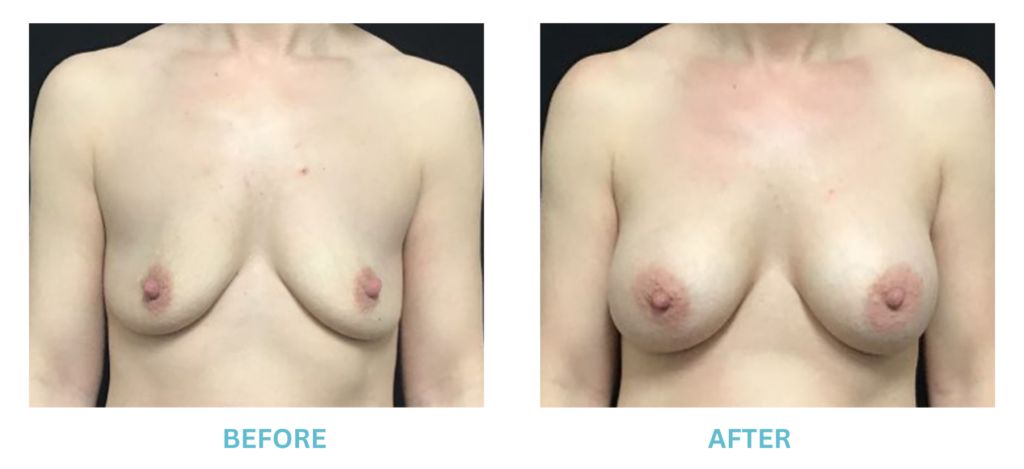
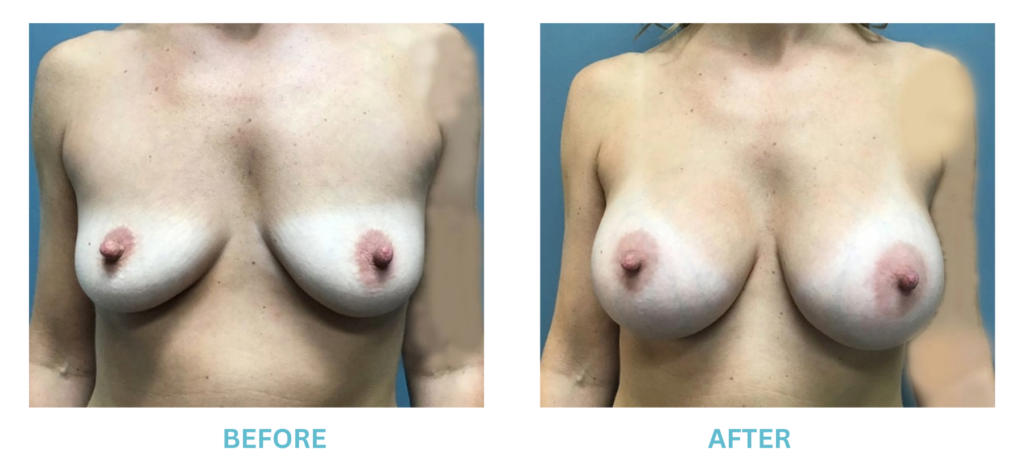
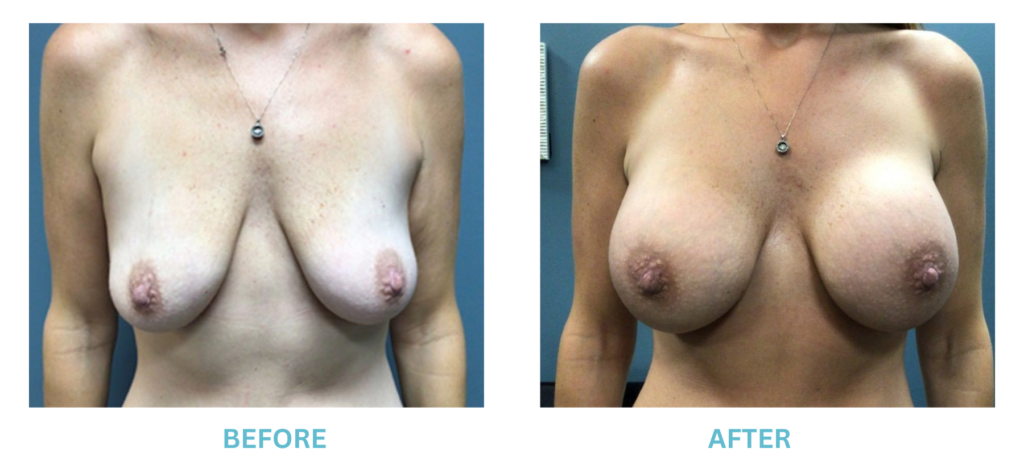
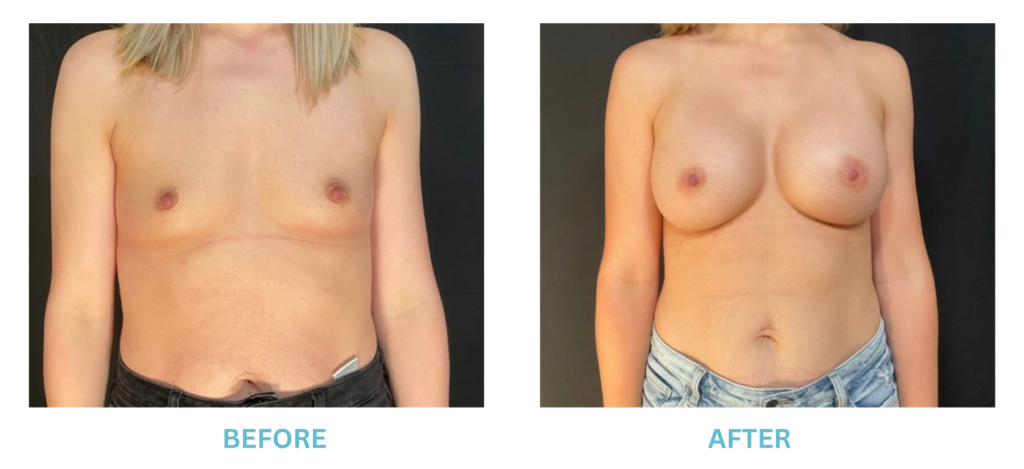
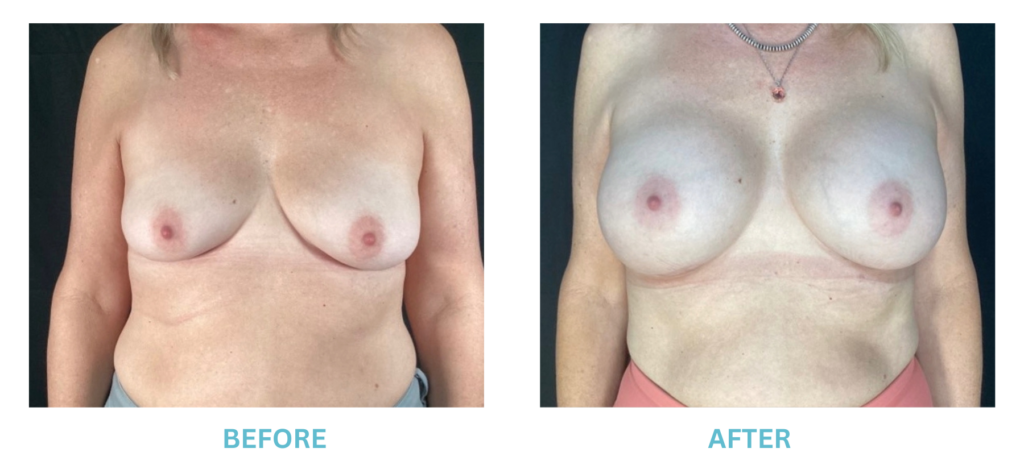
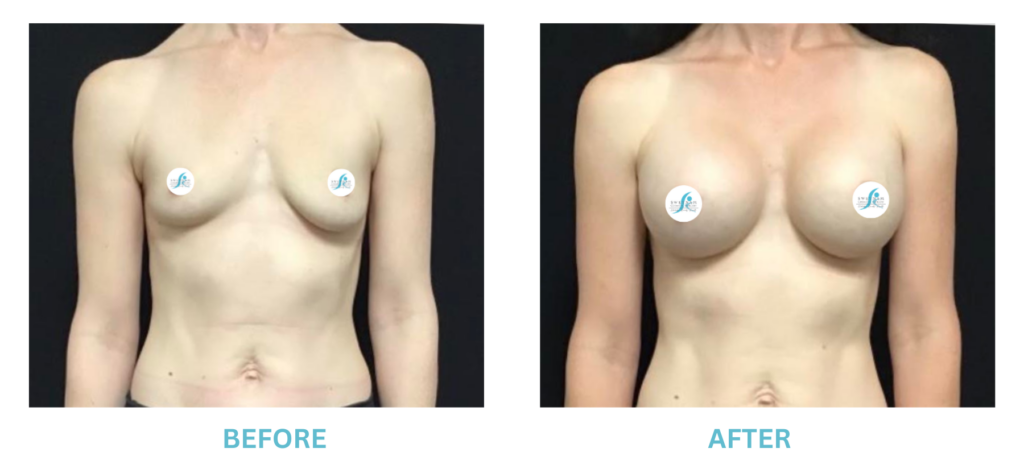
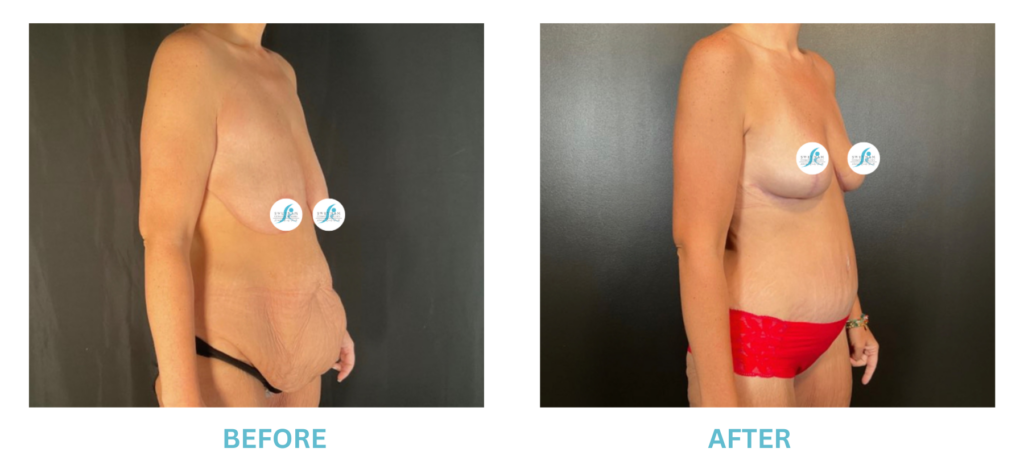
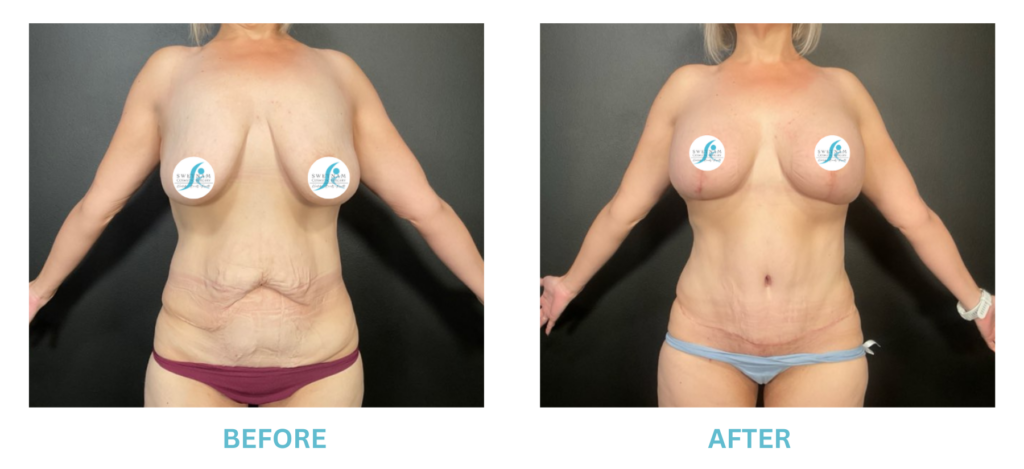
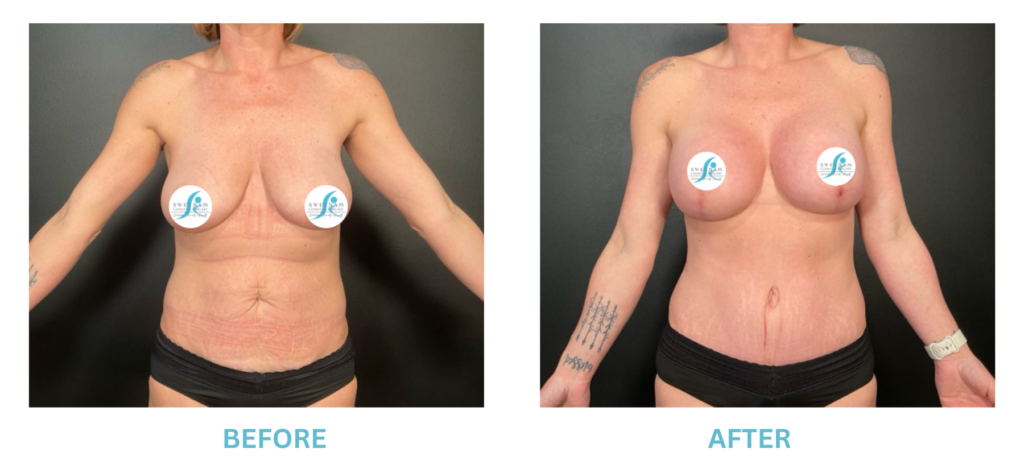
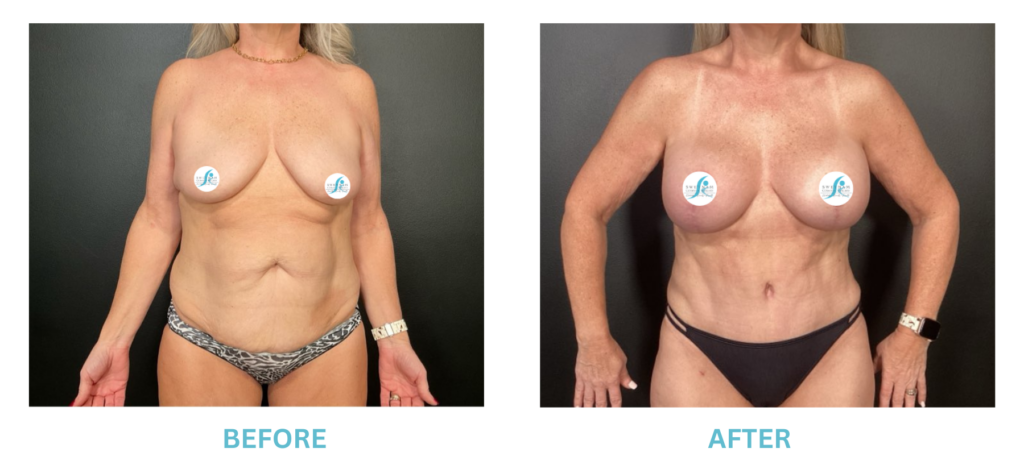
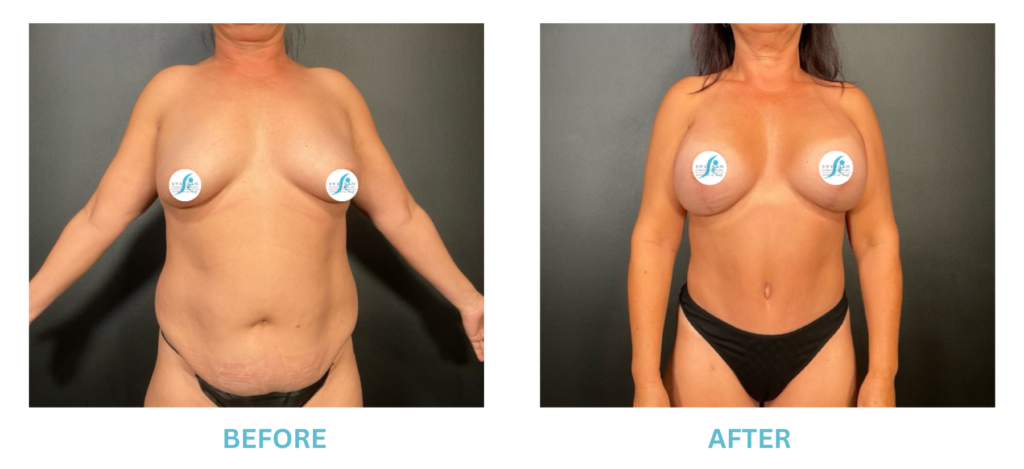
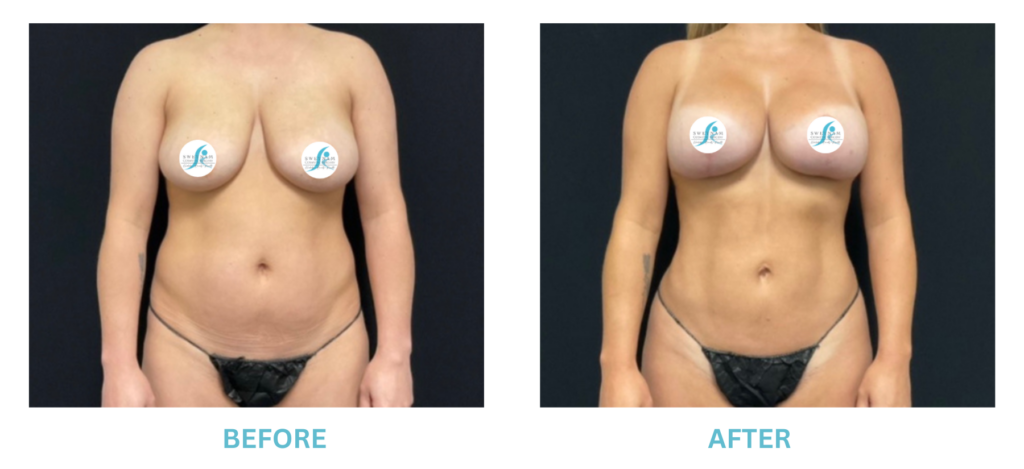
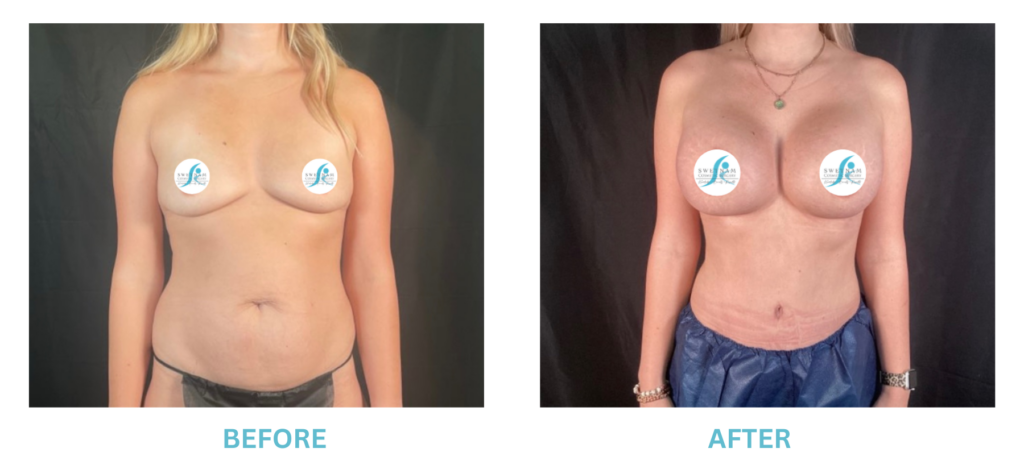
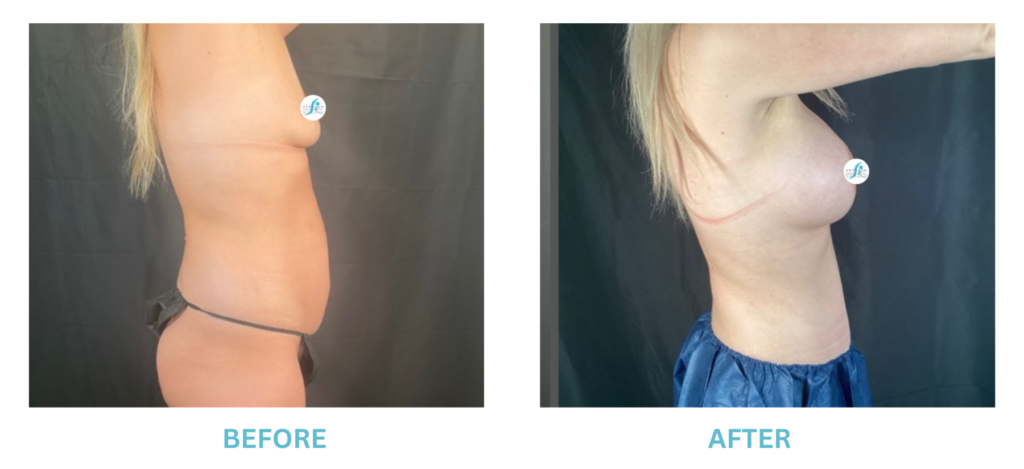
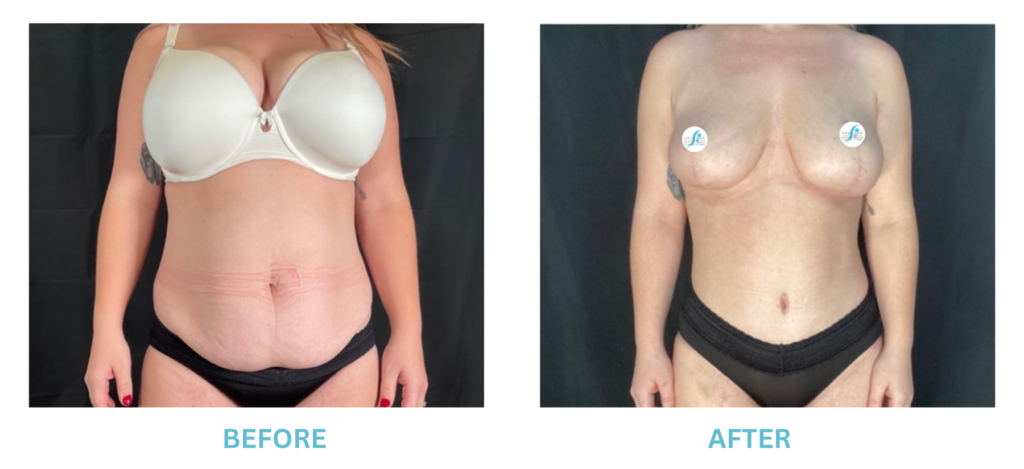
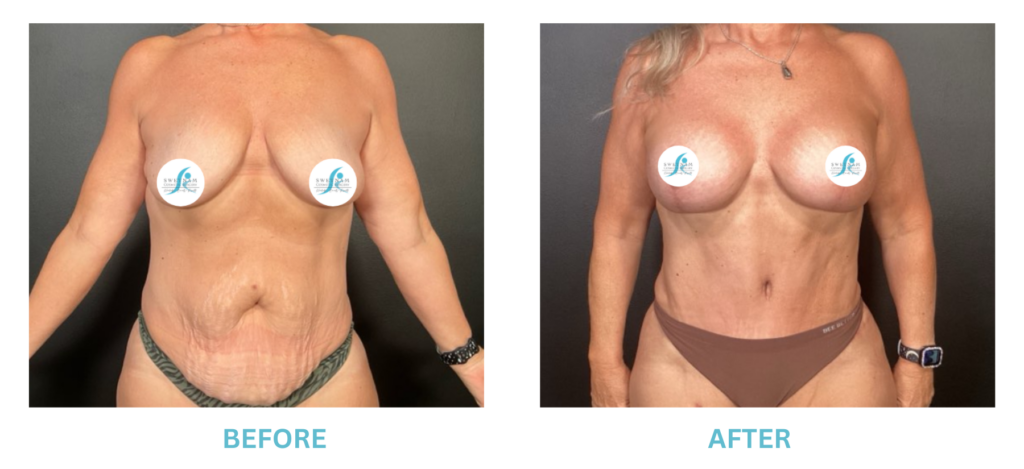
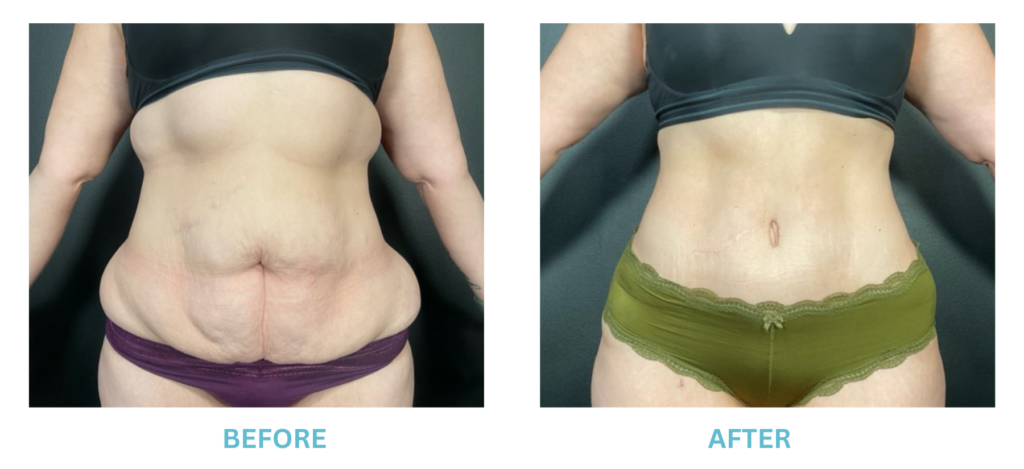
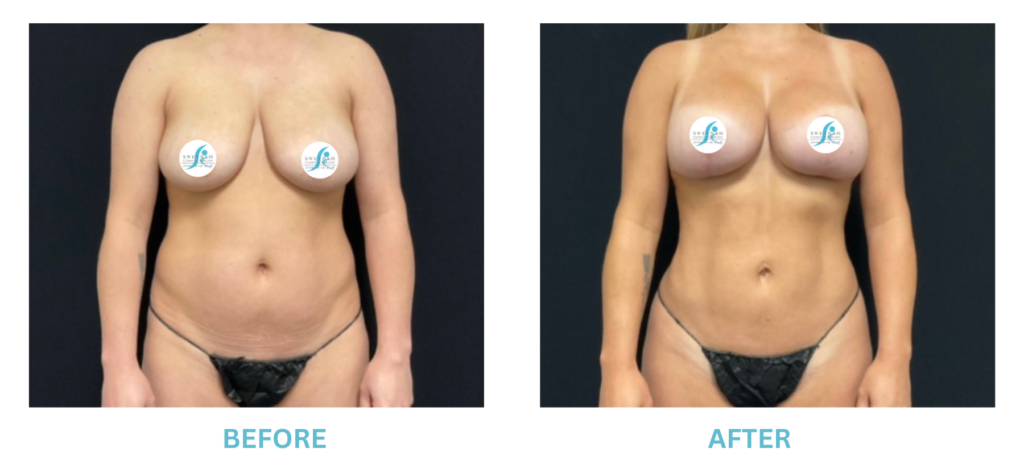
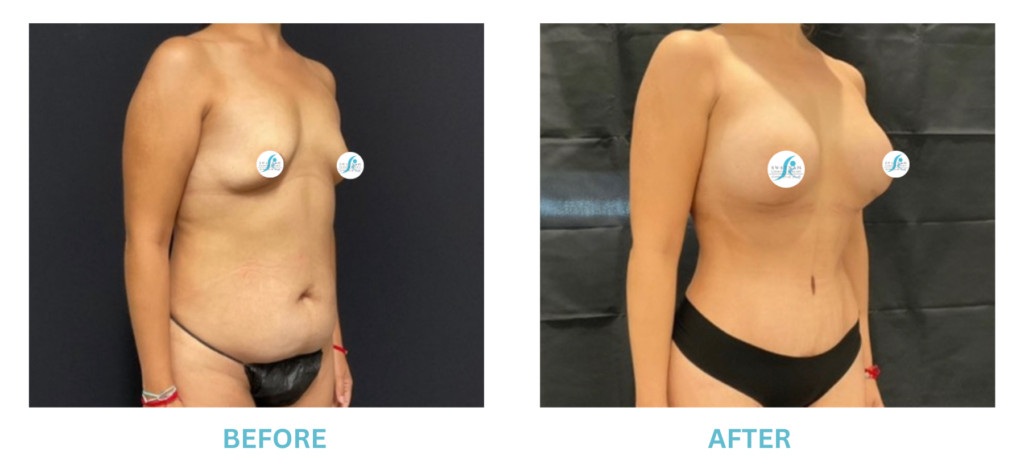
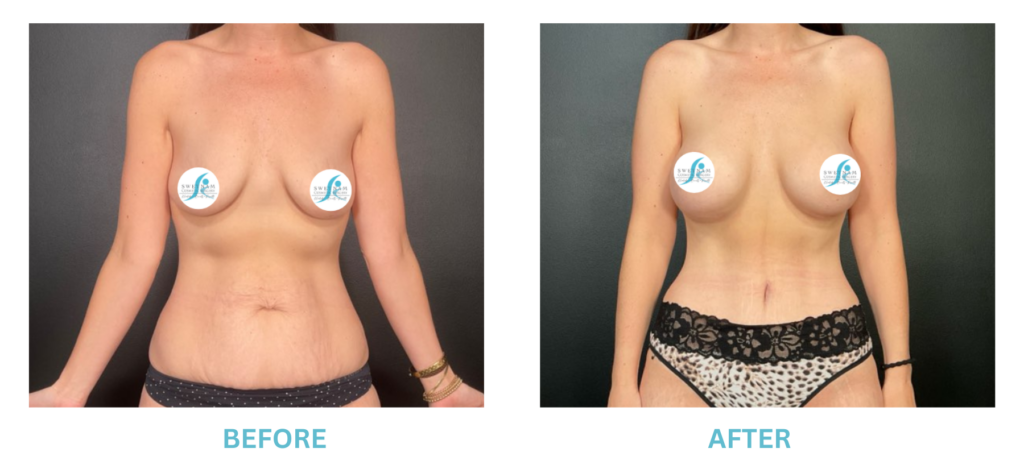
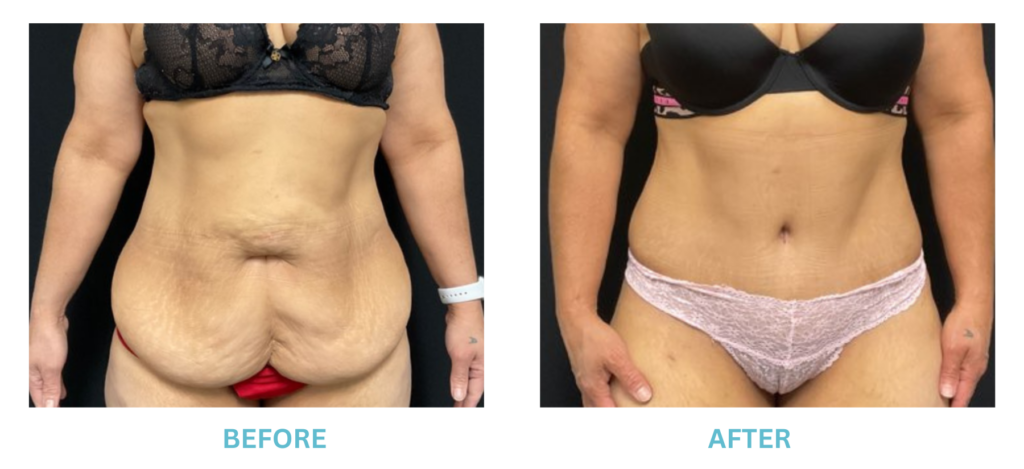
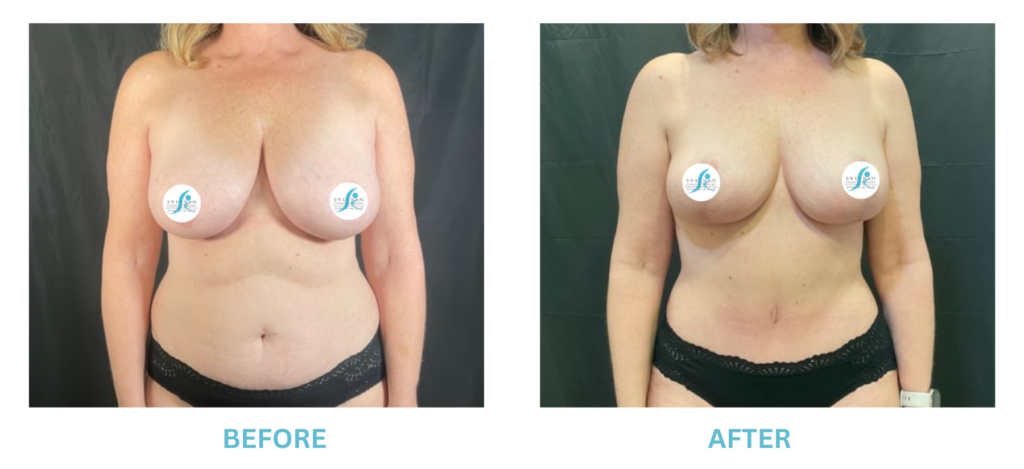
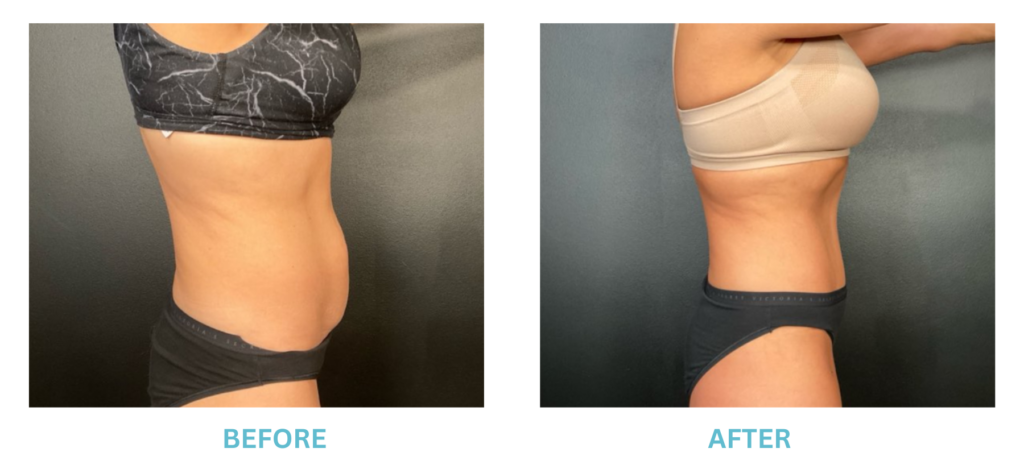
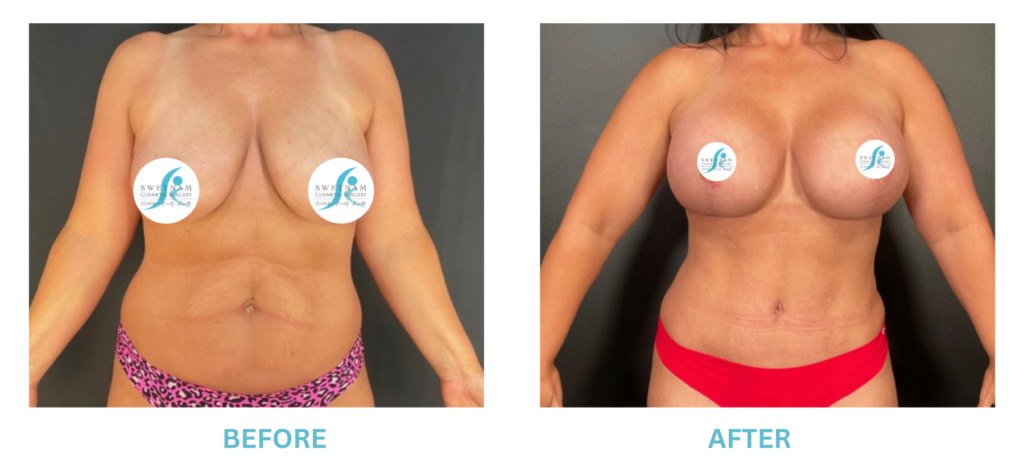
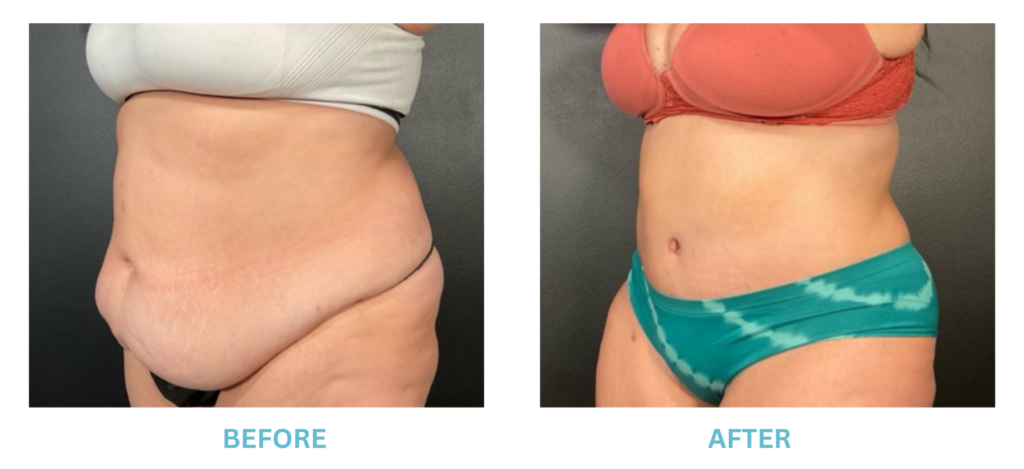
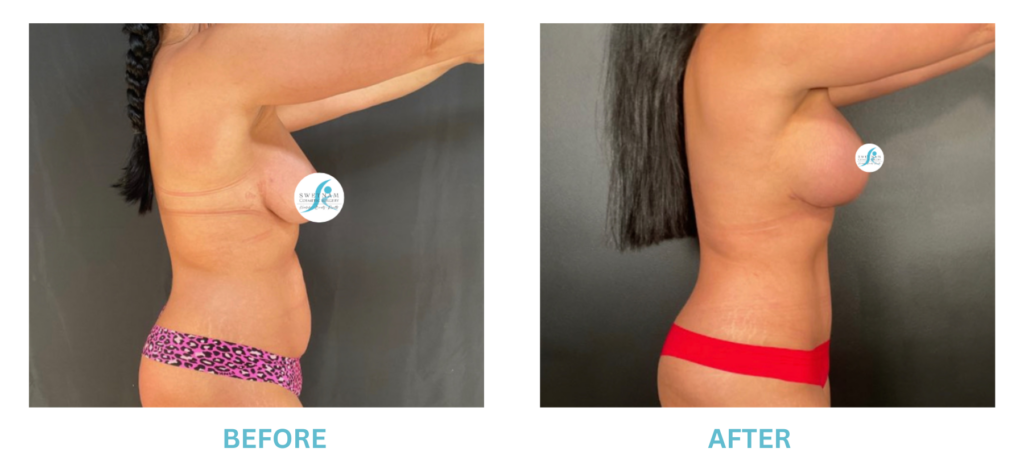
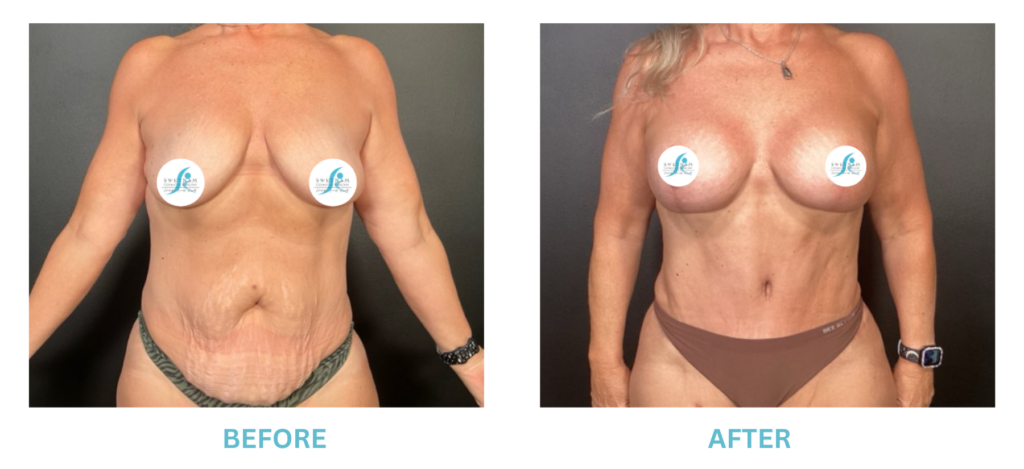
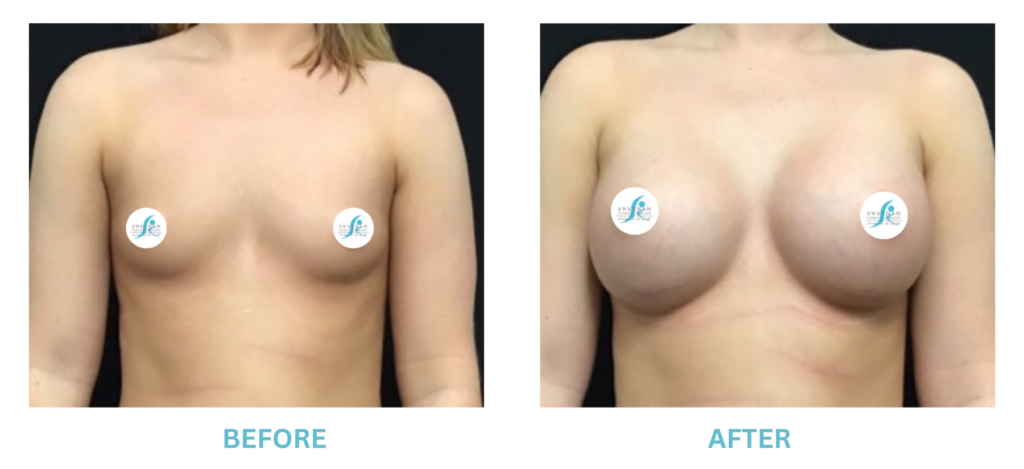
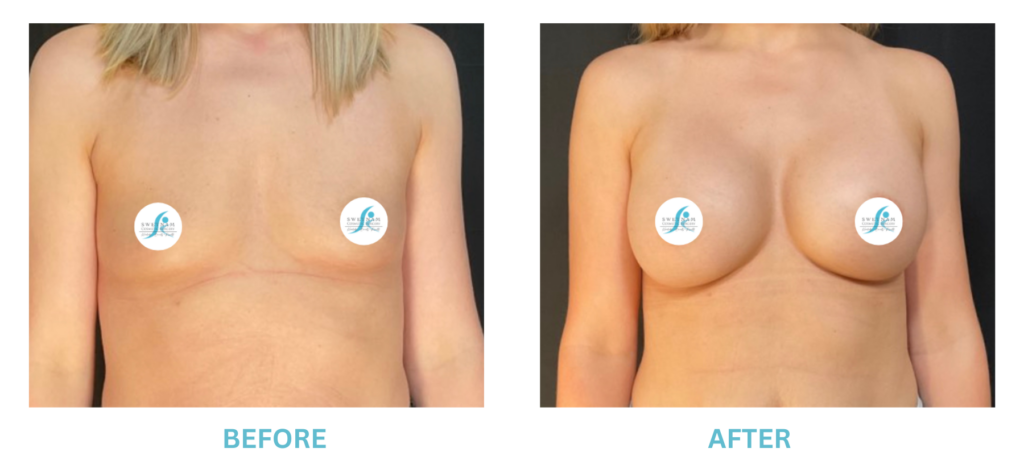
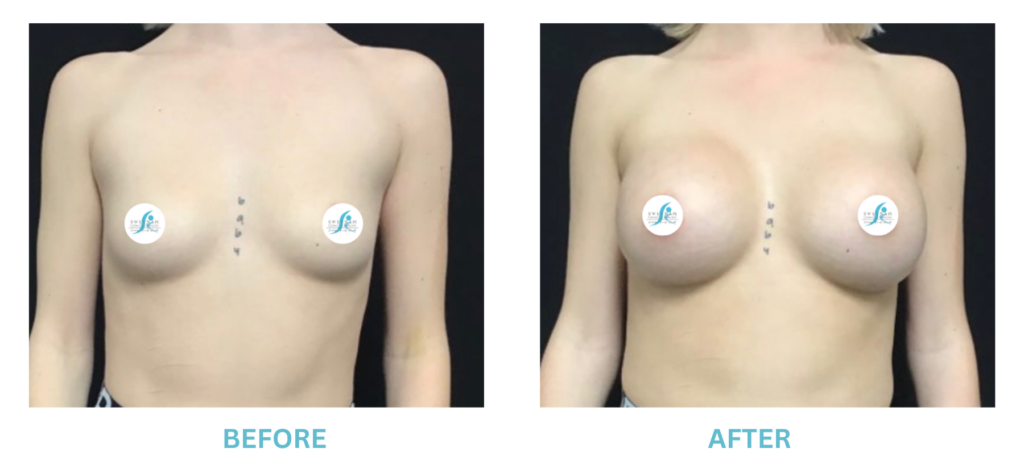
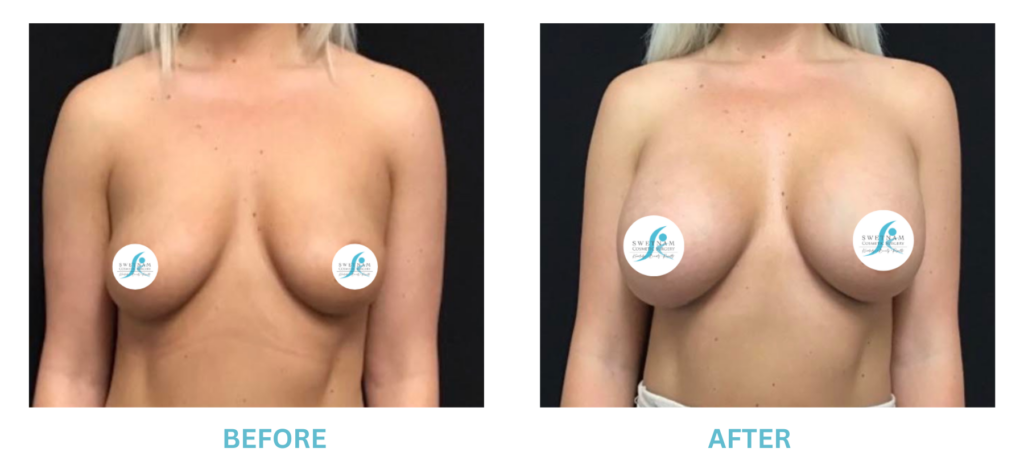
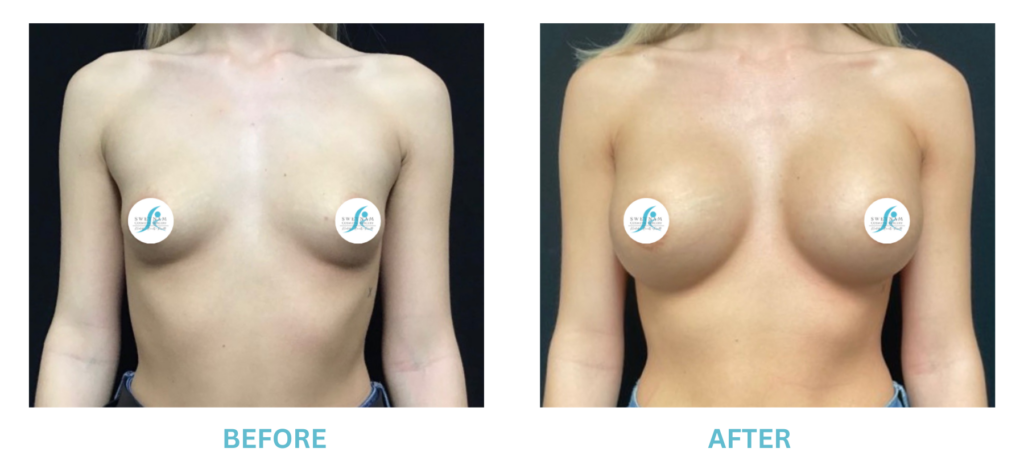
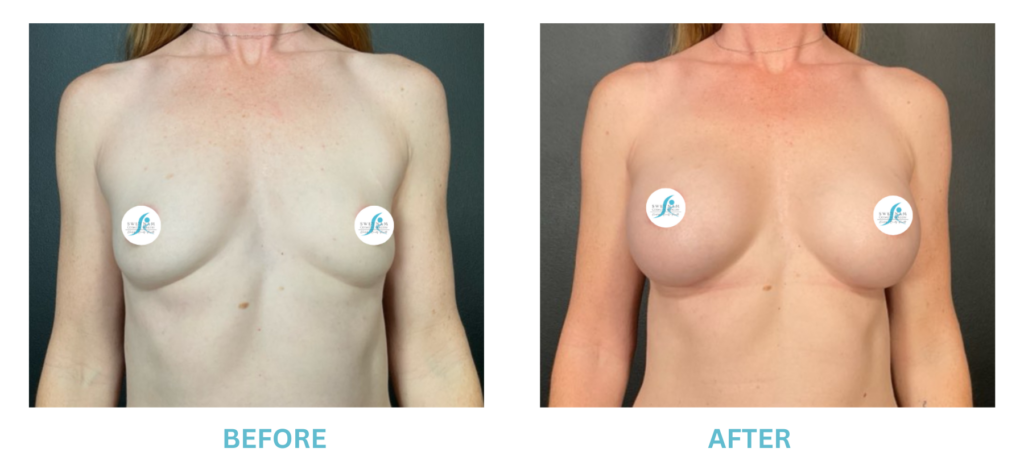
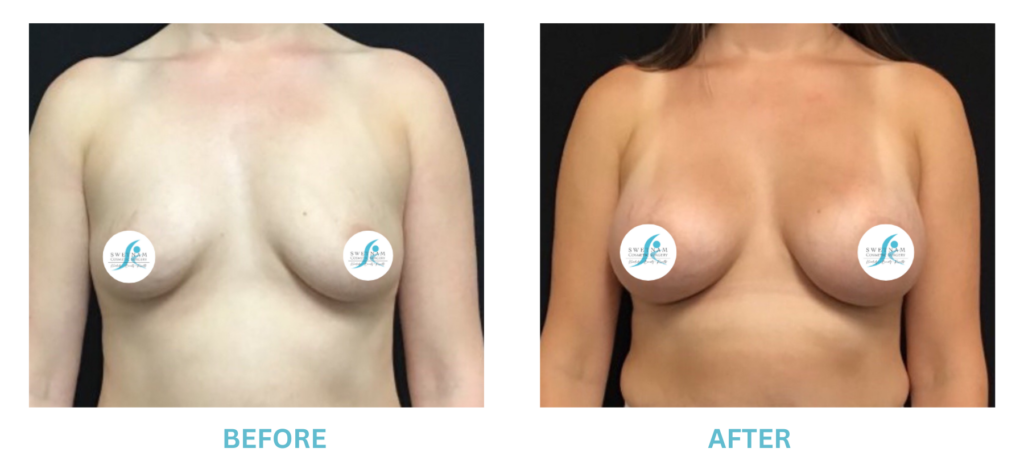
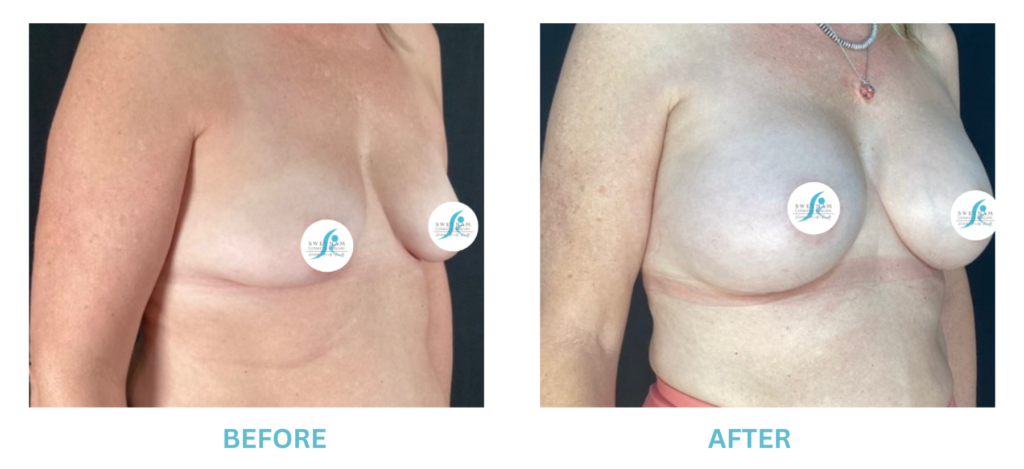
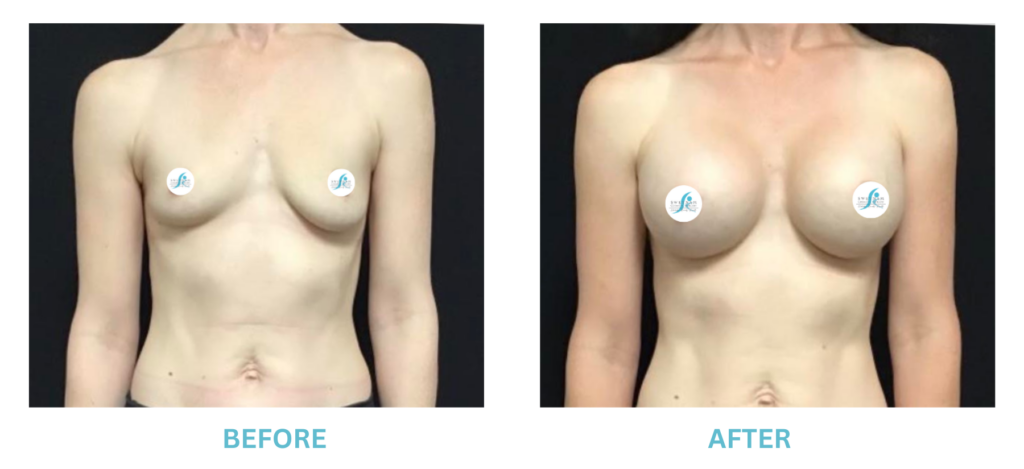
Breast Implants and the FDA
I am sure many of you have seen or heard the FDA warnings recently on breast implants. I have written several blogs and posted several papers from the ABCS about this in the past several months. If you have not read these, you can find them on my Swetnam Cosmetic Surgery Facebook page or this blog post.
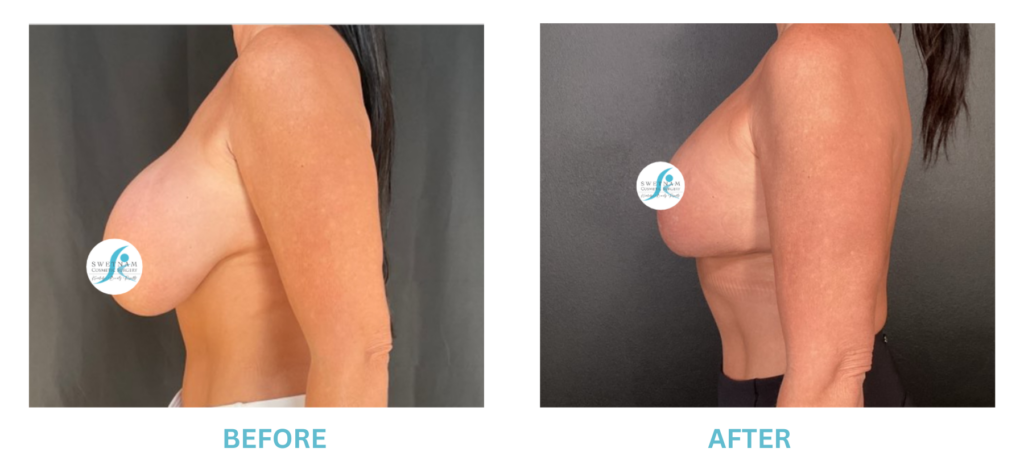
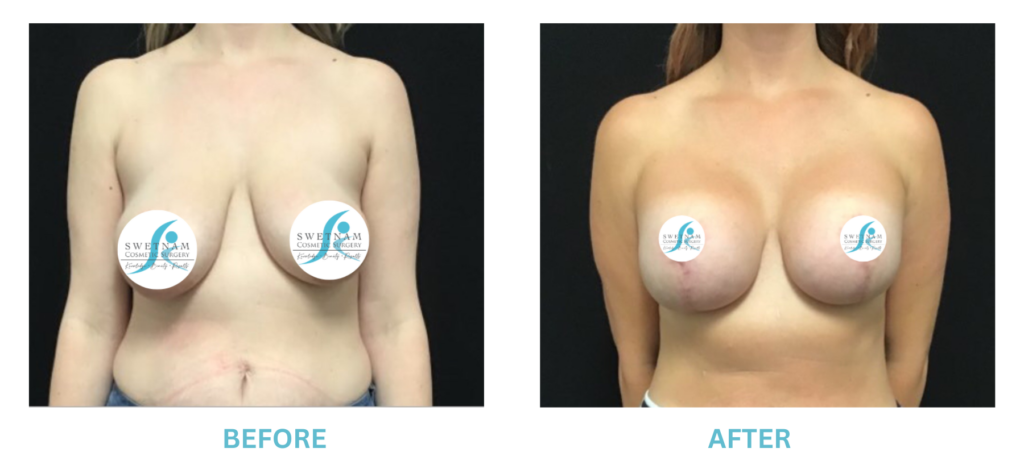
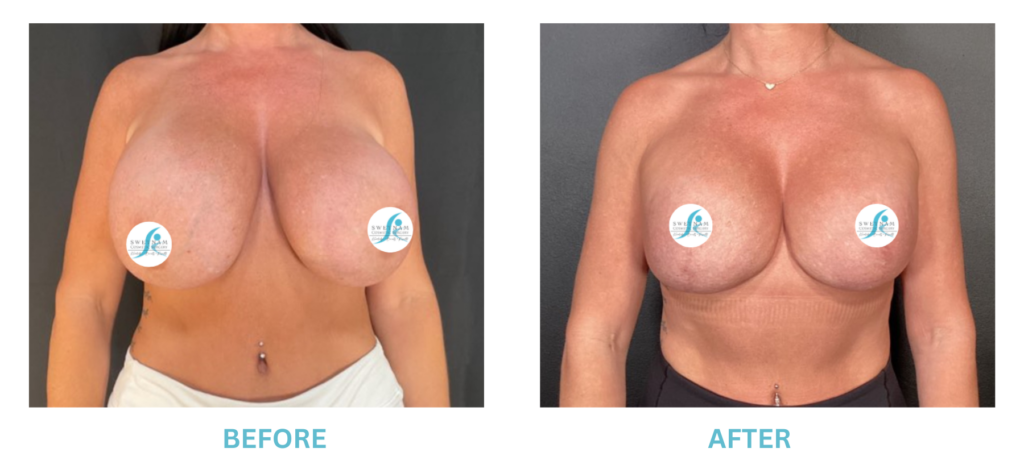
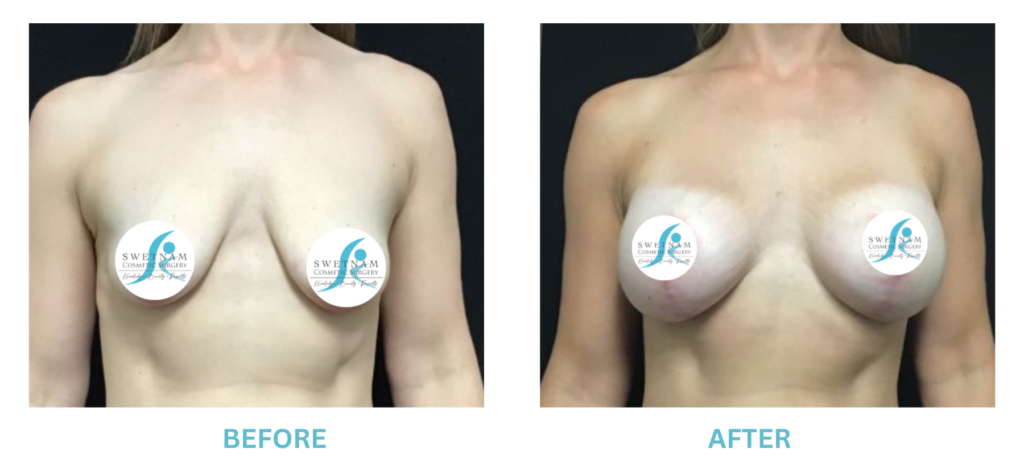
The FDA is concerned that patients do not understand or are not being told the risks of breast augmentation with implants. In particular, the incidence of BIA-ALCL (a rare form of lymphoma associated with Allergan Textured implants), breast implant syndrome (another rare condition where vague symptoms with no other obvious cause except a silicone implant, cured by removing the implants), that implants are not permanent and may be periodically need to be replaced. The FDA also recommends a “checklist” for all patients to cover potential implant issues.
I applaud the FDA for taking these steps. At Swetnam Cosmetic Surgery we have always used a checklist to go over these issues. We have always used smooth implants exclusively, and I have always believed Breast implant syndrome is a reality and treated it as such.
If you are interested in breast implant surgery, are having issues with your current breast implants, or just have questions, please call or come see me at Swetnam Cosmetic Surgery, 479-966-4174.
Jeff Swetnam, MD